⇦ Back to Livestock and Feedstuff Management Home
¶ Introduction
Nutrient composition can vary tremendously between different feeds and forages. Even within a single feed ingredient, there is potential for the nutrient composition to vary significantly.
Laboratory analysis and testing is used to identify the type and concentration of specific nutrients in a feedstuff. The results are used to improve animal nutrition and animal production. Providing adequate nutrition is key to maintaining a healthy, productive and profitable livestock operation.
The first step is to collect a sample or series of samples that accurately represent the feed or forage. A “bad”, non-representative sample may be worse than no sample at all. Other Crop Files describe sampling procedures for various types of feeds and commodities.
There are four primary methods for evaluating feedstuff and forage quality: (1) physical observation and laboratory methods, including: (2) chemical analysis, (3) near-infrared spectroscopy, and (4) in-vivo/in-vitro digestibility.
Samples must be properly prepared before laboratory analysis. Sample preparation of feeds and forages often involves drying and grinding, which mixes and homogenizes the sample. Samples that cannot be dried must be homogenized by other means.
¶ Physical Observation
Sight, smell, and touch can be useful indicators of feed value, but can be misleading. A physical evaluation alone is rarely able to predict the eventual animal performance. Evaluation of the physical characteristics of a feedstuff also depends on the individual perception and experience of the person conducting the evaluation.
Color, odor, leafiness, and texture can be good indicators of hay quality. For example, musty and foul odors can indicate lower quality due to deterioration in storage. Some livestock prefer a soft, pliable texture. Table 1 contains an example of sensory criteria useful to evaluate alfalfa hay.
Stem size, leaf retention, and color help verify the age of cutting. Brown color and “sweetish” smell may indicate heat damage during storage. Physical observations like these can be useful for evaluating feed quality, but there is no way to accurately measure them. Only a laboratory analysis can provide an accurate estimate of the true nutritional value.
¶ Table 1. Sensory Criteria (Visual Appearance, Touch, Smell) Used to Evaluate Alfalfa Hay |
 |
| Maturity stage | Look for the presence of seed heads (grass forages) or flowers or seed pods (legumes), indicating more mature forages. |
| Leaf-to-stem ratio | Look at forage and determine whether the stems or leaves are more obvious. Good-quality legume forages will have a high proportion of leaves, and stems will be less obvious and fine |
| Color | Color is not a good indicator of nutrient content, but bright green color suggests minimal oxidation. Yellow hay indicates oxidation and bleaching from sun, and hay will have lower vitamins A and E content |
| Foreign objects | Look for presence and amounts of inanimate objects (twine, wire, cans, etc.), weeds, mold, or poisonous plants |
| Texture | Feel stiffness or coarseness of leaves and stems. See if alfalfa stems wrap around your finger without breaking. Good-quality hay will feel soft and have fine, pliable stems. |
| Odor | Good quality hay will have a fresh mowed grass odor. No musty or moldy odors. |
¶ Chemical Analysis
Chemical analysis (commonly called “wet chemistry”) involves determining nutrients by chemically reacting or extracting important compounds in a laboratory to determine their concentration in the feedstuff. Wet chemistry methods include a variety of chemical and enzymatic procedures. Analysis may include use of specific temperatures and instrumentation to determine the final nutrient composition.
Chemical methods can directly measure the quantities of compounds associated with an essential nutrient, but may tell us little to nothing about availability. Even so, chemical analysis can provide fairly accurate predictions of animal performance, because the research used to develop nutrient requirements used chemically tested feedstuffs.
Wet chemistry can provide a practical approach to feed analysis by making direct determinations of moisture (water), carbohydrates (fiber, starch, sugar), nitrogen (crude protein), ether extract (fat), and ash (minerals). The basic flow of wet chemistry determinations is illustrated in Figure 1.
¶ Figure 1. Basic Work Flow of Feedstuff Analysis Procedures
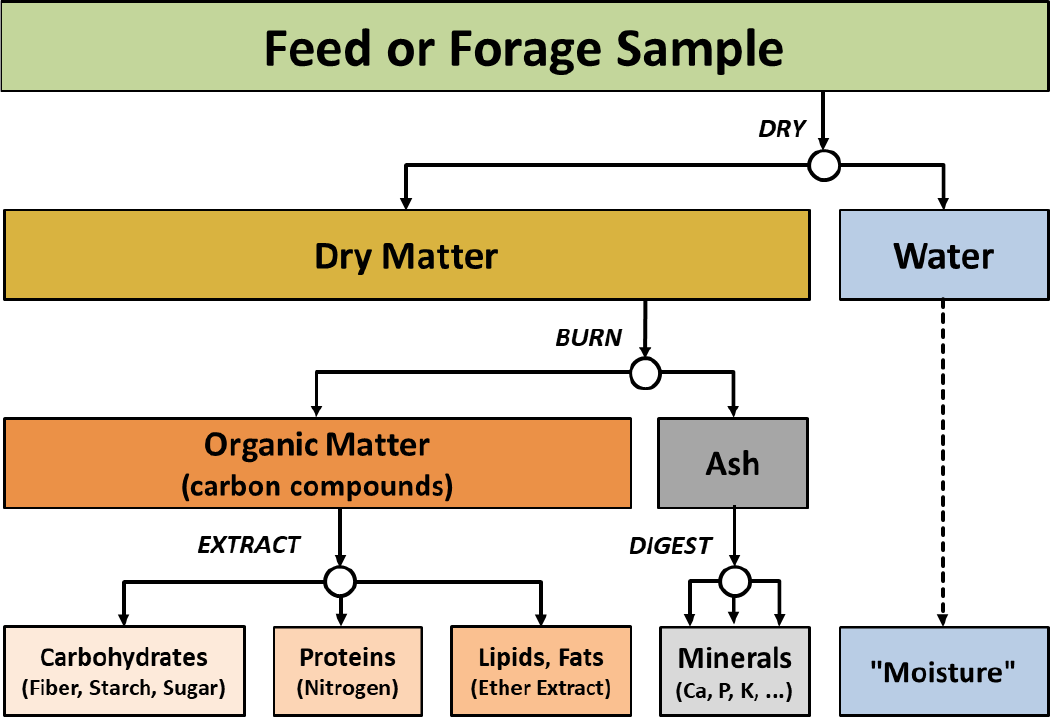
Most wet chemistry methods and procedures have been standardized by various organizations. This includes the Association of American Feed Control Officials (AAFCO), National Forage Testing Association (NFTA), and others. These associations often provide proficiency programs (“check” programs) that evaluate and score analytical quality to help assure consistent results between laboratories and improve quality control.
¶ Near-Infrared Reflectance Spectroscopy (NIRS)
Wet chemistry analysis is considered the "gold standard" for feed testing. Analysis using near-infrared reflectance spectroscopy (NIRS) is a simpler and less expensive method with a short turnaround time. NIRS employs nearinfrared light rather than chemicals. Feeds can be analyzed in less than 15 minutes using NIRS, compared to hours or days for chemical methods.
Forage or grain samples are dried and ground, then exposed to infrared light in a spectrophotometer. Each major organic component of the sample absorbs and reflects near-infrared light differently. The reflected infrared radiation is converted to electrical energy and fed to a computer for interpretation. By measuring these different reflectance characteristics, the NIRS unit and a computer determine the quantity of these components in the feed sample.
Specific nutrients can be detected by NIRS because reflectance spectra from samples of known nutrient values—established by wet chemistry procedures—are programmed into the computer. When a similar feed sample is evaluated by NIRS, the computer compares the wavelength reflections from the sample, then matches them to previously tested samples.
The type of feedstuff or forage being submitted for analysis must be matched to the right feed library. Proper calibration is all important in NIRS analysis. The calibration set that is used must be developed from an adequate number of wet chemistry samples similar to those being analyzed by NIRS. These samples must be carefully collected and stored and consistently dried, ground, and mixed prior to analysis.
NIRS analysis generally has high accuracy in measuring crude protein and fiber fractions compared to wet chemistry for many feedstuffs, but is less accurate in measuring feed mineral content. Although NIRS analysis is faster and less costly, there is debate on the complete accuracy and interpretation of the analysis.
Results might not be accurate if a sample contains a mixture of different forage species or if the feed library does not have entries that are calibrated with the same species from the same area.
Even with feed library calibrations, NIRS may not be appropriate for some feedstuffs. For example, NIRS will adequately measure moisture, percent crude protein, calcium, and phosphorus in distillers grains (feed byproducts from the ethanol industry).
However, NIRS does not do an adequate job of measuring the energy (as total digestible nutrients, TDN) content of the distillers grains. TDN may be estimated using acid detergent fiber (ADF). ADF measures cell wall content of a feedstuff. Because distillers grains are high in fat, NIRS will underestimate their energy content.
NIRS can determine forage nutritional content rapidly and less expensively than wet chemistry methods, when properly prepared and calibrated.
¶ Figure 2. Illustration of Process to Develop Calibration Equations for NIRS Feed Analysis
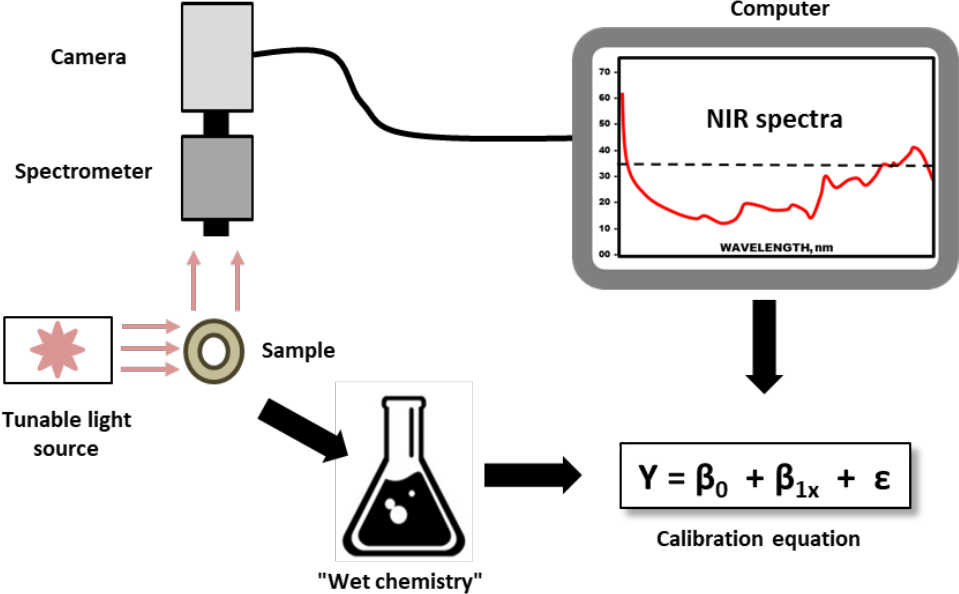
¶
¶ In Vivo/In Vitro Digestibility
Chemical methods identify total nutrient concentration, but do not necessarily provide good information about availability or digestibility. Biologic or enzymatic methods provide a different nutritional perspective to feed analysis. These methods extract certain fractions of the feedstuff or ration that may help to better understand just how the animal will interact with its diet.
Relative digestibility is often determined using in-vivo or in-vitro methods. These procedures are relatively expensive and take several days to complete.
“In-vivo” procedures are more often used in research because they require test animals, take days or weeks to run, and are expensive. One in-vivo procedure involves incubating a feed sample by placing a small, closed nylon bag in the rumen of a cow that has been surgically fitted with a cannula (porthole-like device that allows access to the rumen). The material lost from the bag is measured after different lengths of time, providing data on the rate of feed degradation.
“In-vitro” procedures are conducted in test tubes to simulate the animal’s digestive system, essentially an artificial rumen (see Figure 3). The feed sample is placed in the tube with some type of liquid, typically rumen fluid or an enzyme solution (like pepsin). The sample is digested for one or more specified time periods at a standard temperature.
¶ Figure 3. University of Minnesota In-Vitro ("Artificial Rumen") Fermentation Study System

https://researchfeatures.com/exploring-microbial-digestive-dynamics-ruminants-in-vitro/
In-vitro analysis results are usually entered into a computer model to calculate various parameters important to nutritional management and production.
The amount of sample that disappears during the digestion period is used to estimate the concentration of digestible dry matter and the relative digestibility. Though useful, the dry matter disappearance provides little or no information about the quantity of specific nutrients that become available to the host animal.
Further analysis may include methods to measure volatile fatty acids, microbial biomass production, or fermentation gas release to isolate specific nutrients or nutrient fractions that may be involved in digestion and metabolism.
¶ References
Van Saun, R. 2013. Determining Forage Quality: Understanding Feed Analysis. Penn State Univ. Ext. https://extension.psu.edu/determining-forage-qualityunderstanding-feed-analysis
Rasby, R.J., et. al. 2008. NebGuide G1892 - Understanding and Using a Feed Analysis Report. Univ. of Neb. Coop Ext. accessed 19July2022 https://extensionpublications.unl.edu/assets/html/g1892/build/g1
Horrocks & Valentine. 1999. Forage Quality: The Basics in Harvested Forages. Brigham Your Univ. , Provo UT. pg 17-47.