⇦ Back to Soil Resource Management Home
¶ Introduction
The primary cause of growth problems in sodic soils is due to excess exchangeable sodium which causes dispersion of soil aggregates. This can result in crusting, loss of soil permeability, and restricted root growth. Elevated soil pH or potentially toxic concentrations of certain ions may cause nutritional problems for some plant species.
¶ Soil Exchange Complex
The surfaces of microscopic clay particles are occupied by positively charged ions (or “cations”) of various compounds. These surfaces are referred to as the “exchange complex” and the associated ions are called “exchangeable” ions.
Calcium and magnesium ions that dominate the exchange complex encourage individual clay particles to “flocculate” or clump together. This forms soil aggregates (granules) which provide pore spaces that allow water and air movement through the soil.
“Sodic” soils are those where sodium ions become a dominant cation on the exchange complex. Sodium ions gradually cause “deflocculation” (dispersion) of soil aggregates back to individual clay particles. When exchangeable sodium levels are high enough, there are not enough exchangeable calcium and magnesium ions to offset aggregate dispersion.
Sodium may also cause certain clay minerals to swell, effectively squeezing soil pore spaces shut. The soil "runs together", air and water permeability are reduced, and crop growth is restricted.
¶ Exchange Reactions
Exchange reactions refer to movement of ions between the soil exchange complex and soil solution. The exchange surfaces of the individual clay particles are essentially covered with negative charges. Clay particles are microscopic - less than 2 microns in size (about 0.00008 inch). Individual clay particles normally bind together in crumb-like aggregates of various sizes and shapes.
The "soil solution" is the soil water and the dissolved materials found in and around these soil aggregates.
¶ Sodic Soils
The primary cause of growth problems in sodic soils is due to excess exchangeable sodium which causes dispersion of soil aggregates. This can result in crusting, loss of soil permeability, and restricted root growth. Elevated soil pH or potentially toxic concentrations of certain ions may cause nutritional problems for some plant species.
¶ Soil Exchange Complex
The surfaces of microscopic clay particles are occupied by positively charged ions (or “cations”) of various compounds. These surfaces are referred to as the “exchange complex” and the associated ions are called “exchangeable” ions.
Calcium and magnesium ions that dominate the exchange complex encourage individual clay particles to “flocculate” or clump together. This forms soil aggregates (granules) which provide pore spaces that allow water and air movement through the soil.
“Sodic” soils are those where sodium ions become a dominant cation on the exchange complex. Sodium ions gradually cause “deflocculation” (dispersion) of soil aggregates back to individual clay particles. When exchangeable sodium levels are high enough, there are not enough exchangeable calcium and magnesium ions to offset aggregate dispersion.
Sodium may also cause certain clay minerals to swell, effectively squeezing soil pore spaces shut. The soil "runs together", air and water permeability are reduced, and crop growth is restricted.
¶ Exchange Reactions
Exchange reactions refer to movement of ions between the soil exchange complex and soil solution. The exchange surfaces of the individual clay particles are essentially covered with negative charges. Clay particles are microscopic - less than 2 microns in size (about 0.00008 inch). Individual clay particles normally bind together in crumb-like aggregates of various sizes and shapes.
The "soil solution" is the soil water and the dissolved materials found in and around these soil aggregates. When a salt is dissolved in water they dissociate into "ions", some positively charged (cations) and some negatively charged (anions)>
There are many ions involved in the exchange reactions common to sodic soils, but we are mainly concerned with three cations: calcium (Ca+2), magnesium (Mg+2), sodium (Na+); and four anions: bicarbonate (HCO3-), carbonate (CO3-2), sulfate (SO4-2), and chloride (Cl-).
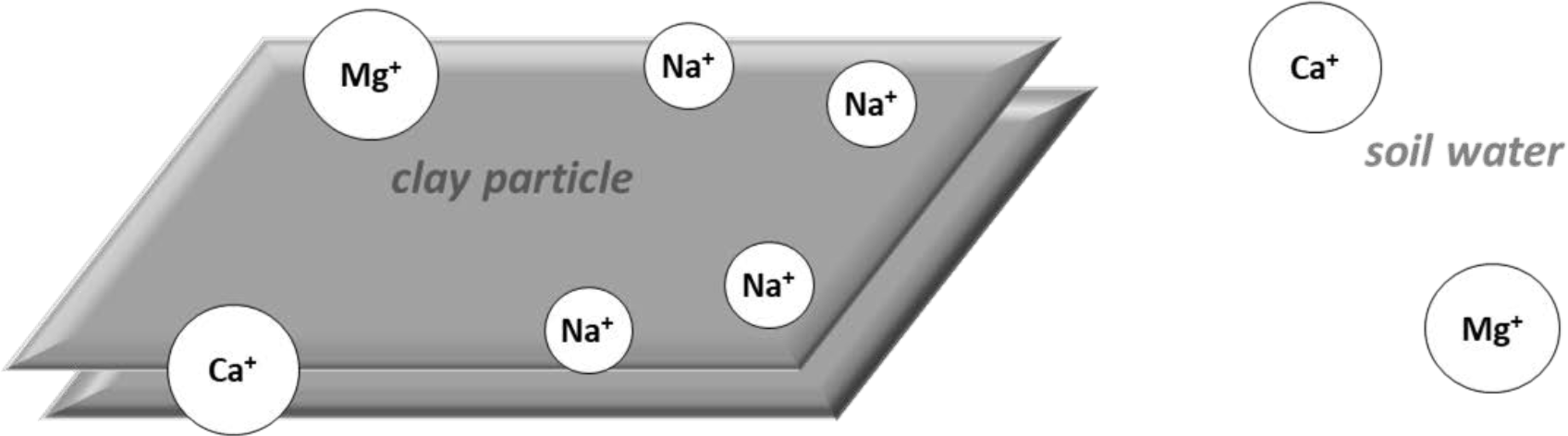
There is a normal exchange of cations between the soil solution and exchange complex, as shown in Figure 1a. Ions seek to maintain chemical equilibrium between the solution and exchange complex. Calcium and magnesium ions are more strongly adsorbed on the exchange complex than are sodium ions.
As water is removed from the soil by evaporation, ions in solution become more concentrated. When the concentration is high enough, the ions in solution reach their solubility limits. They combine to precipitate (or "salt out") into their solid forms (Figure 1b). Calcium and magnesium ions precipitate sooner than do sodium ions. Bicarbonate and carbonate ions precipitate sooner than do sulfate and chloride ions.
Calcium and magnesium typically precipitate first, forming relatively insoluble carbonates. This is followed by precipitation as sulfates and finally as chlorides. As the calcium and magnesium ions are precipitated and removed from solution, calcium and Mg ions move off the exchange complex into the soil solution to maintain equilibrium (Figure 1c.).
Sodium ions in solution (which have not precipitated) move onto the exchange complex and occupy sites formerly held by calcium and magnesium ions (Figure 1d.). The exchangeable sodium percentage is likely to continue increasing if the soil water is continually recharged by water with elevation sodium concentrations. This may be naturally occurring or from irrigation water applications.
¶ Loss of Exchangeable Calcium and Magnesium
This chemical precipitation causes a net loss of calcium and magnesium ions from the exchange complex. If the precipitated calcium and magnesium salts remain in the soil, they could theoretically redissolve and replace sodium on the exchange complex when the soil was rewetted. Calcium and magnesium carbonates are so insoluble, that for practical purposes they are lost from the exchange system. Calcium and magnesium sulfate salts may remain, but are can be rather easily leached away.
The loss of calcium and magnesium ions causes a relative increase of sodium ions in solution. Increasing sodium ions in solution also increases the potential to increase the proportion of exchangeable sodium ions. This increases the sodium concentration as a percentage of ions found on the cation exchange complex.
The sodium adsorption ratio (SAR) of the soil water or irrigation water becomes important diagnostic tool in estimating potential for an increased exchangeable sodium percentage (ESP). The SAR expresses the relationship of the calcium and magnesium ions to the sodium ions found in soil solution. An SAR value of less than about 60% to 70% the value of the ESP indicates a high proportion of calcium and magnesium ions in solution that would be available to replace those that might be lost by precipitation. An example would be an SAR value of 5 with an ESP of 10% in the accompanying soil. An SAR value greater than about 80% to 90% of the ESP indicates a high proportion of sodium ions in soil solution that are likely to exchange with calcium and magnesium ions lost from the exchange complex. An example would be an SAR of 14 and an ESP of 15%.
¶ Clay Swelling and Dispersion
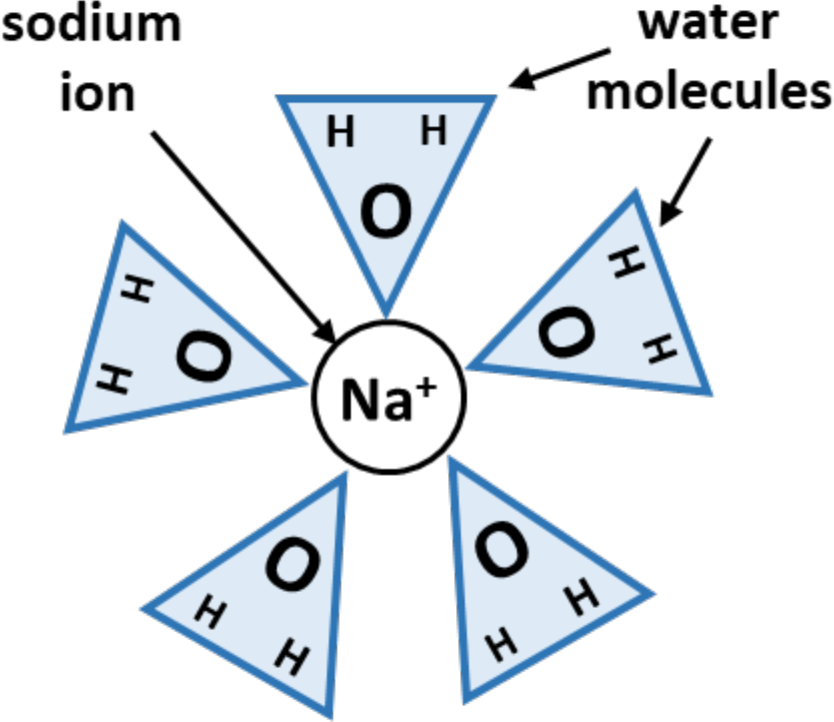
Sodium ions are expected to affect soil structure when ESP exceeds about 10% in a typical medium textured soil. As sodium ions occupy sites on the exchange complex, they to form a "shield" of water molecules around themselves as illustrated in Figure 2. This concept is called the “hydrated radius”. The effective radius of the hydrated ion is much larger than the unhydrated ion. Hydrated sodium ions are surrounded by an average of five water molecules. This gives the sodium ions a tendency to repel each other.
¶ Figure 2. Representation of a Hydrated Sodium Ion
Individual clay particles begin to repel one another when sodium ions occupy enough sites on the external layers of the exchange complex. When the ESP reaches 10% to 15%, the repelling force of the sodium ions is strong enough to overcome the forces holding clay particles together as aggregates. Aggregates break down and disperse into individual clay particles. These dispersed particles begin to plug and block the small pore spaces in the soil. This slows water and air movement through the soil.
When the ESP exceeds 25% to 30%, sodium ions can occupy sites on inner layers the clay minerals. Water molecules associated with the sodium ions (i.e., the “shield”) cause individual clay particles to swell. As clay particles swell the existing pore spaces get smaller. Soil structure deteriorates rapidly at this point because of the combined effects of swelling and dispersion. Dispersed clay particles are already blocking and plugging soil pore spaces. Swelling reduces the size of the pores and makes them easier to plug.
¶ Soil Dispersion Effects
Breakdown of soil structure is the primary management problem with sodic soils. Soil permeability decreases as the ESP increases. The rate of air and water movement can be effectively zero in severely sodic soils. The poor soil structure and root system restrictions resulting from this dispersion make it difficult for the crop to obtain enough water, air, or nutrients from the soil to maintain proper growth. Seedling establishment and growth is difficult. Root growth is restricted and root systems stay small.
Clay content, organic matter content, and clay mineralogy all influence the point at which dispersion occurs. Soils with a high clay content tend to disperse more rapidly than those of lower clay content. Clay or silty clay soils may start dispersing with ESP values of 6% to 8%. Sandy soils may not show obvious dispersion symptoms until the ESP exceeds 15% to 20%.
Organic matter helps to maintain flocculation, so a high organic matter content delays dispersion. Montmorillonite or smectite clay minerals swell more when wetted than do illite or kaolinite clay minerals. Dispersion and swelling of the clay particles occur sooner in soils with these types of clay minerals.
¶ Soil pH Effects
Many sodic soils are highly alkaline, having a soil pH of 8.5 or greater. Exchangeable sodium hydrolyzes and forms sodium hydroxide (NaOH) in soil solution. NaOH reacts with dissolved carbon dioxide (CO2) to form sodium bicarbonate (NaHCO3). This raises the soil pH to 9 or 10.
The soil pH typically drops to more normal levels when the excess sodium is removed during remediation.
Plants growing in sodic soils often show symptoms of iron deficiency chlorosis because of the elevated soil pH. Iron chlorosis is not actually an iron deficiency within the plant, but an inability of the plant to properly metabolize available iron. Some researchers believe that chlorosis occurs because the excess bicarbonate taken up from the soil solution may block movement of iron from veins to interveinal areas. Iron is not available to complete photosynthesis, so portions of the leaf tissue between the veins turn yellow and may die. The elevated pH in sodic soils can also result in deficiencies by reducing solubility and availability of other plant nutrients, like phosphorus and zinc.
Not all sodic soils are alkaline. There are a few "degraded" sodic soils with a pH of 7 or lower. Some degraded soils are actually acidic. These soils did not contain the calcium and magnesium salts to replace the ions removed from the exchange complex during soil formation. Therefore, sodium ions (Na+) and hydrogen ions (H+) become dominant on the exchange complex. The high percentage of exchangeable hydrogen ions causes the acid soil condition.
¶ Potentially Toxic Ions
Some sodic soils may have chloride and boron ions present in excessive amounts in the soil solution. These ions can be absorbed by the root system and accumulate within the plant to excessive levels. This accumulation may cause nutritional imbalances or toxicity symptoms in certain sensitive plants. Refer to Crop File 4.02.014, Toxic Ions in Salt Affected Soils, for a more detailed discussion.
¶ Figure 1a. Lons at Equilibrium on Exchange Complex and in Soil Solution
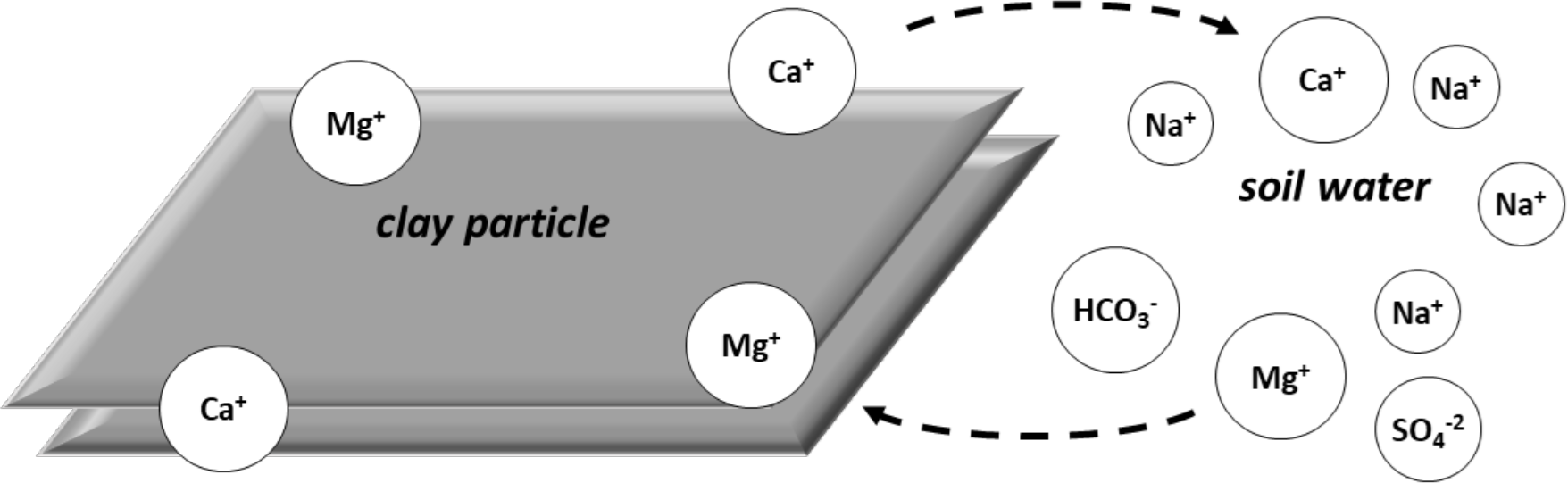
¶ Figure 1b. Soil Dries; Calcium and Magnesium Precipitate and Are Removed From Soil Solution
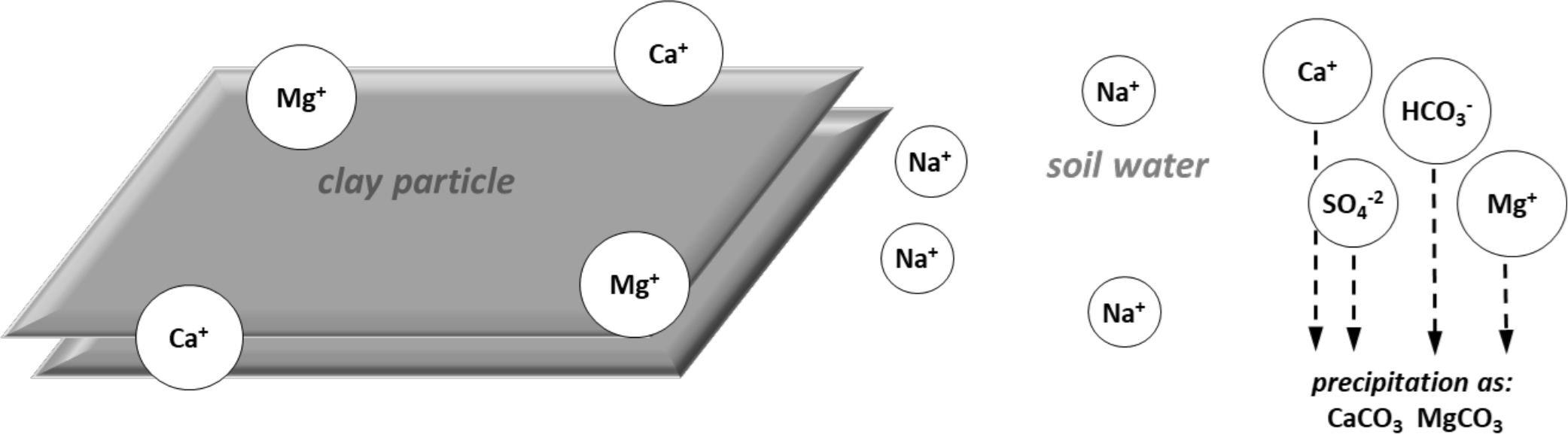
¶ Figure 1c. Lons on Exchange Complex and in Soil Solution Seek New Equilibrium
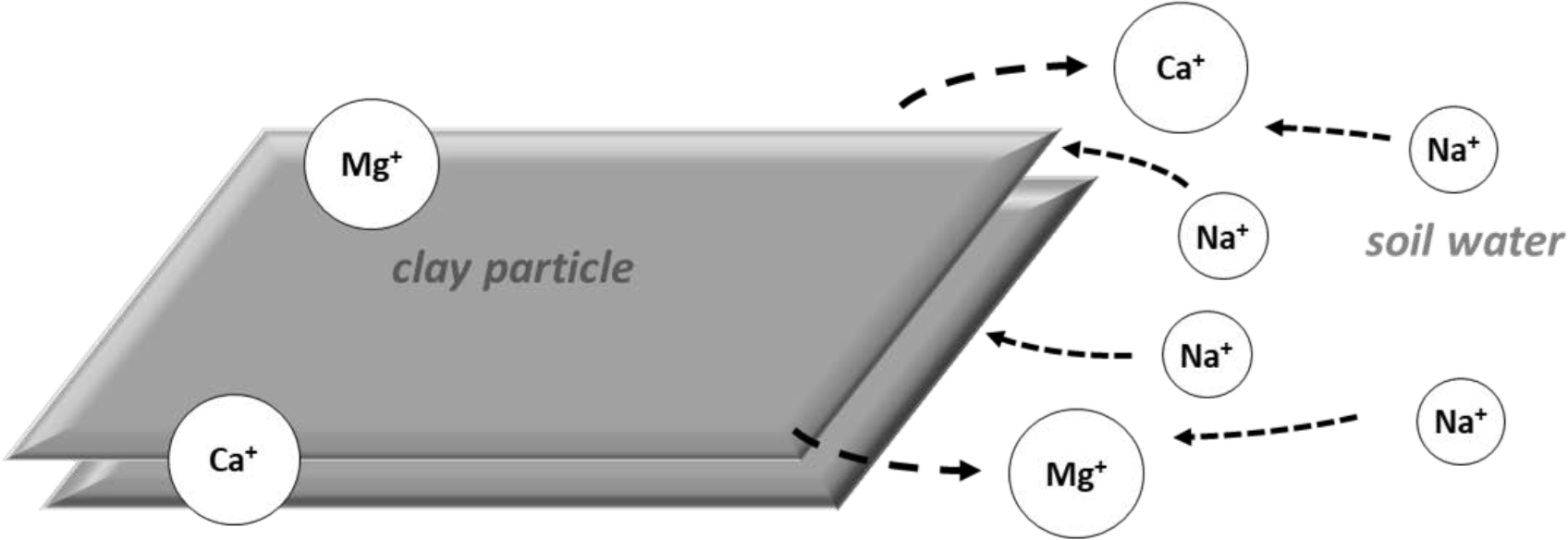
¶ Figure 1d. New Ion Equilibrium Is Established; Soil Gradually Becomes Sodic