⇦ Back to Soil Resource Management Home
¶ Introduction
What we call “soluble salts” develop as minerals in the soil and underlying materials break down during the weathering process. Soluble salts are found in all soils, but if they accumulate to excessive levels the physical properties and productivity of that soil can be affected. Salt‐affected soils normally occur as small, irregular areas in a field. They range in size from a few square feet to an acre.
Soils are called "saline" when the total concentration of salts (or “salt load”) interferes with plant water uptake and affects crop growth. Soils are called "sodic" when the proportion of sodium salts to other salts (like calcium and magnesium) are high enough to degrade the soil structure. “Saline‐sodic" soils have both a high total alt concentration and a high proportion of sodium salts.
¶ Formation Factors
Salts can accumulate in soils when four conditions exist:
- a climate with high evaporation rates,
- a water source,
- a source of soluble salts, and
- an internal drainage restriction that prevents good downward movement of water.
Salts tend to accumulate in soils of arid or semi‐arid regions because rainfall is low and evaporation rates are high. Soluble salts seldom accumulate in humid region soils because there is usually adequate precipitation to leach salts below the root zone.
Weathering of soil minerals is the source of soluble salts. As soil minerals break down, elements like silica, aluminum, and iron often recombine to form soil clays. Other elements like calcium, magnesium, potassium, and sodium do not form new clays, but remain as soluble salts.
Over time water from precipitation will dissolve these soluble salts and transport them to an underlying formation (like an aquifer) or to a body of surface water. All water sources contained dissolved salts (minerals): some very dilute; some more concentrated. Other conditions then determine if salts will accumulate in a soil from a specific water source.
Sometimes this salt‐charged water feeds a seasonal or a permanent water table. If the water table is less than five to six feet below the land surface, capillary action can draw water to the surface of medium‐textured and fine‐textured soils.
Capillary rise is less in coarser‐textured soils, about three to four feet. Capillary action not only draws water upward, but the dissolved salts as well. Subsequent evaporation removes the pure water, but leaves the salts behind. If the salts are not leached away by rain or irrigation, they have potential to accumulate in the soil.
Shallow water tables, compacted zones, or differential soil layers (sand or clay "lenses") can restrict the free downward movement of water. This prevents the accumulated salts from being leached away.
¶ Floodplains
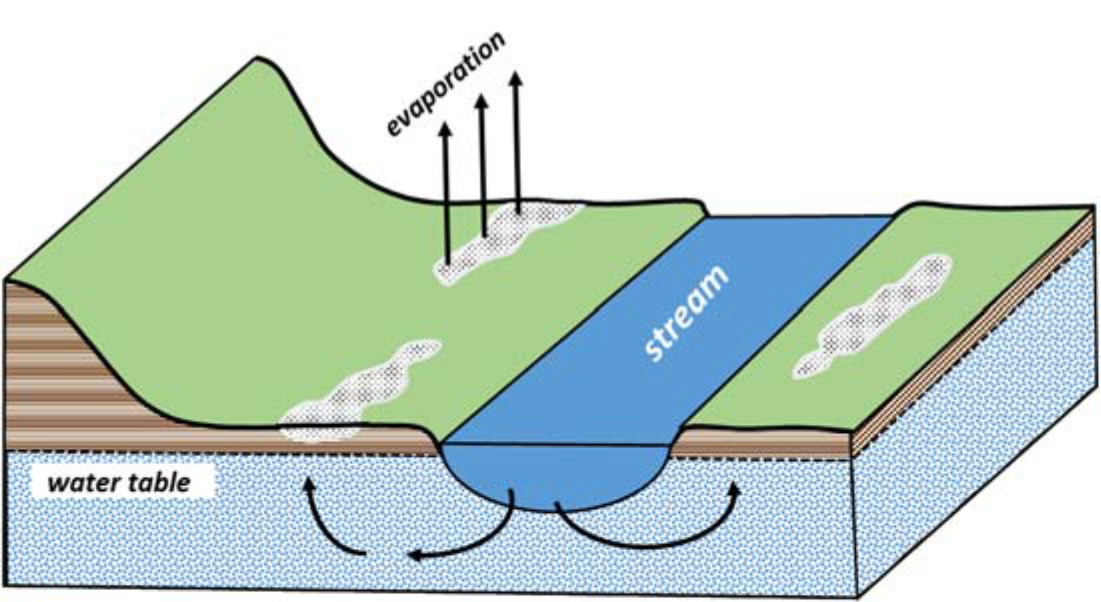
Figure 1 illustrates a common situation that occurs in the floodplains and in the low‐lying areas near rivers, lakes, or streams. In this case, underground movement of water originating from the stream transports dissolved salts as it moves laterally through the soil or through the water‐bearing aquifer formation.
The stream keeps the water table within four to six feet of the soil surface, allowing capillarity, which prevents downward water movement. The capillary effect draws the salt‐charged water upwards. Evaporation removes the water leaving the salts behind in the upper soil profile.
The stream‐fed water table serves for water recharge, as a salt source, and as a drainage restriction. Weather conditions, both short‐term and long‐term, that affect the stream level, evaporation rates, and precipitation will affect the rate of salt accumulation. Thus, the severity of the salinity problem may change through the year or over a period of years. Ponds or unlined canals can be recharge sources in other cases.
¶ Figure 1. Salt-Affected Soil Area Develops in Floodplain From High Water Table
¶ Seeps, Pan Spots
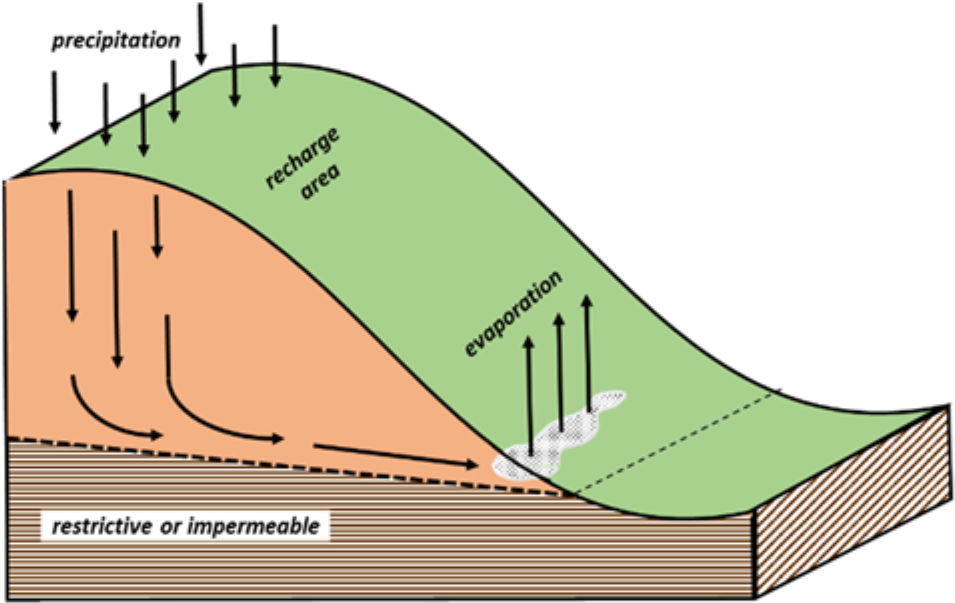
Salt‐affected soils sometimes form in uplands or on hillsides, distant from surface water sources. These areas, often called "seeps" or “pan spots”, can develop downslope from a water recharge areas as illustrated in Figure 2.
These recharge areas may not be immediately obvious. Fallow areas or windbreaks often act as water recharge areas. They trap precipitation during the non‐crop season and allow moisture to drain below the normal root zone. The moisture percolates through the soil, dissolving salts from the soil as it goes.
The salt‐charged water moves downward until it reaches a restrictive layer. This layer can be impermeable bedrock or can be a soil layer that is two to three textural classes different than the overlying soil (e.g., sandy over clayey or clayey over sandy). This is enough to restrict easy downward movement of the soil water resulting in “perched” water. The water flows downslope along the surface of this layer until it comes close to the land surface. Capillary action may draw it to the surface or it may exit directly as a natural spring or a seep.
Salts accumulate at the surface during periods of high evaporation. These salt‐affected areas retain moisture and dry out more slowly than the rest of the field. Consequently, they are often tilled when wet. This compacts both the salt‐affected area and the normal soil on the edges. Water percolation through the newly compacted soil is slowed, so water remains on the surface longer. This allows salts accumulate around the edges in the normal soil. The salt‐affected area gradually gets Producers using good irrigation larger over time.
¶ Figure 2. Salt-Affected Soil Develops in Upland Area From Restrictive Subsurface Layer
¶ Irrigation Water
Salt‐charged irrigation water can affect a soil rather quickly. An acre‐foot of good quality water may easily contain one‐half ton of dissolved salts. Poor quality water may contain four to five tons of salt per acre‐foot of irrigation. If a restrictive layer is present, like a clay pan or compacted zone, water does not readily percolate to leach away the accumulated salts.
Producers using good irrigation management may unknowingly increase soil salt levels. Producers have improved irrigation efficiency by adopting technologies that apply water only to meet crop demand. No extra water is available to leach salts below the root zone. Salt levels can build quickly in dry years because water is removed quickly by high crop demand and because proportionately larger amounts of dissolved salts are applied during irrigation.
Seepage from unlined canals provides water which helps feed existing tables. Water tables can rise above normal levels and move leached salts upward. Excess runoff that collects at the lower end of surface‐irrigated fields can recharge small saltaffected areas.
¶ Other Sources
Salt‐affected soils can develop overnight from brine or salt water spills which accompany oil drilling activities. The degree of damage may be slight or quite severe depending on salt concentrations and amount of water spilled.
Some salt‐affected soils formed on the remains of ancient sea beds or lake bottoms. As these bodies of water dried up, large amounts of salt were trapped in the remaining deposits. Trapped salts are dissolved and released as these deposits are weathered.
¶ Summary
Soil mineral weathering is a source of soluble salts. Subsurface water moving laterally from one area to another may dissolve and redeposit these salts. When these salts accumulate to excessive levels, normal crop production is affected. The type and severity of the salinity problem depends on the total amount of salt deposited and the salt composition.