⇦ Back to Soil Resource Management Home
¶ Introduction
This Crop File discusses various factors that can affect the cation exchange capacity (CEC) or anion exchange capacity (AEC) of a particular soil. These factors include clay content, clay type, organic matter, cation characteristics, solution composition, and soil pH. Other Crop Files in this series have additional information on this topic.1
¶ A. Sources of CEC
- Clay particles
- Sands, silts, and clays formed from weathered rocks.
- Larger particles gradually weathered and broken down into smaller particles.
- Smaller particles have greater surface area.
- Smaller particle edges are ragged or imperfect.
- Negative charges develop from broken chemical bonds as particles are broken apart.
- Charges develop on particle surfaces and edges. ii. Serve as cation exchange sites.
- Soil organic matter (SOM)
- SOM formed after decomposition and weathering of plant materials.
- Plant materials are primarily cellulose and hemicellulose.
- Decomposed materials used to synthesize humus and humic compounds.
- “Humification” process converts cellulose and hemicellulose-type materials to lignin-type materials and complex proteins
- Results in large, complex, very stable organic compounds (i.e., humus, humic materials
- Humification alters chemical composition and produces numerous potential cation exchange sites.
- SOM has particular affinity to adsorb herbicides due to high CEC.
- Herbicide rates usually higher on soils with higher organic matter levels and greater clay content.
- Soil applied herbicide will have adjusted rates in product label
- CEC does not change readily.
- Final result of climate and weathering ultimately affects soil exchange capacity
- Weathering of mineral and organic parent materials determines texture, clay mineral type, organic matter content, and soil pH
- SOM formed after decomposition and weathering of plant materials.
¶ Table 1. Typical Range of CEC Values For: |
|
| Soil materials (meq/100g): | |
| sand | 1 - 2 |
| silt | 4 - 10 |
| clays kaolinite | 3 - 15 |
| Illite, mica | 15 - 40 |
| chlorite | 20 - 40 |
| montmorillonite, smectite | 80 - 120 |
| vermiculite | 120 - 150 |
| soil organic matter | 150 - 400 |
| Textures (mEq/100g soil): | |
| sands | 3 - 5 |
| loamy sand | 4 - 8 |
| sandy loams | 6 - 12 |
| loams | 15 - 20 |
| silt loams | 15 - 25 |
| clay loams | 15 - 25 |
| sandy clays | 15 - 30 |
| sandy clay loams | 15 - 30 |
| silty clay loams | 20 - 35 |
| clays | 25 - 50 |
| organic soils, mucks ((OM > 20%) | 50 - 100 |
| Plant roots (mEq/100 g of dry tissue): | |
| wheat | 23 |
| corn | 29 |
| bean | 54 |
| tomato | 62 |
¶ B. Clay content (soil texture)
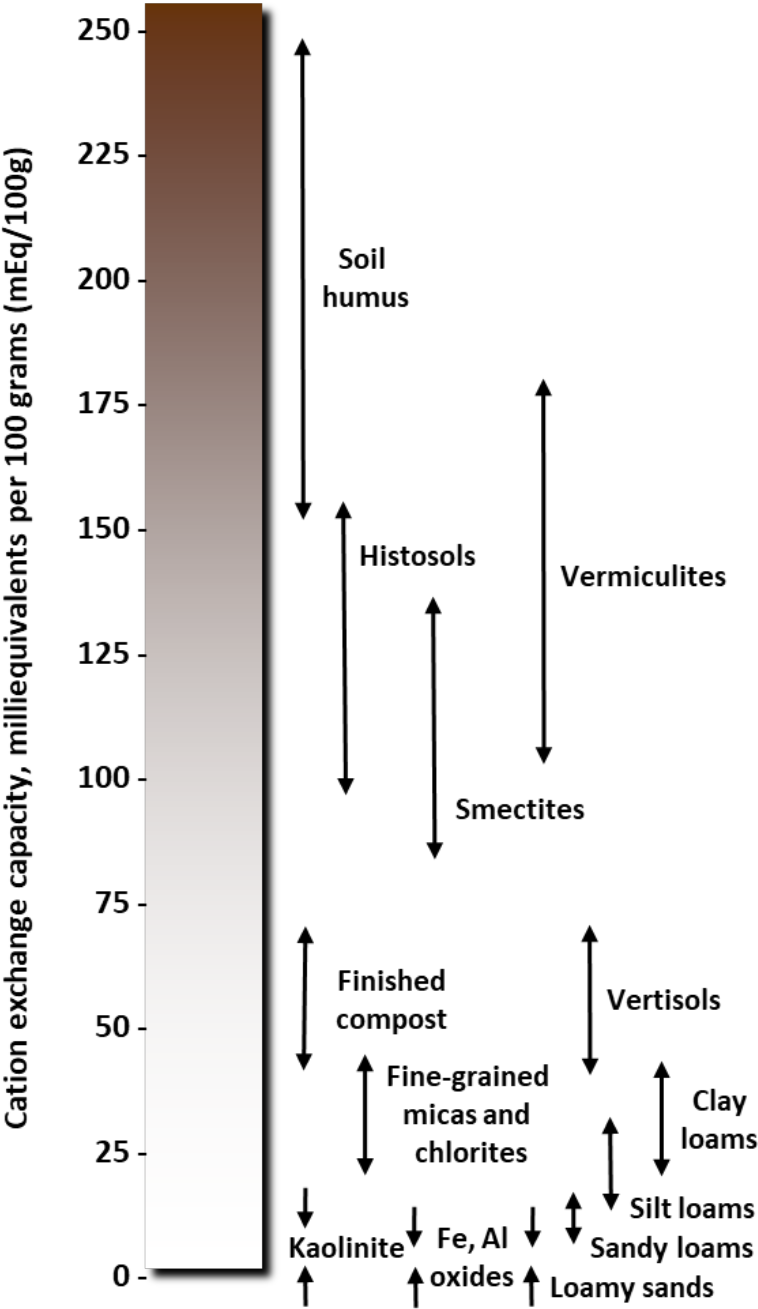
- Soils with higher clay content have higher CEC (see Table 1 and Figure 1).
- Clay particles have high negative charge.
- Is due to many broken edges and large surface area.
- Clay particles have diameter of 2 microns (0.002 mm) or smaller[1].
- Are over 90 billion clay particles per gram. ii. Have surface area of 8,611 square feet per gram.
- Clay particles have high negative charge.
- Sandy soils have lower CEC.
- Sand particles are larger with fewer negative charges.
- Fewer broken edges and small surface area.
- Range of sand particle diameter from 50 to 2,000 microns (0.05 to 2.00 mm).
- Are 90 to 722,000 sand particles per gram.
- Have surface area ranging from 2 to 35 square inches per gram.
- Sand particles are larger with fewer negative charges.
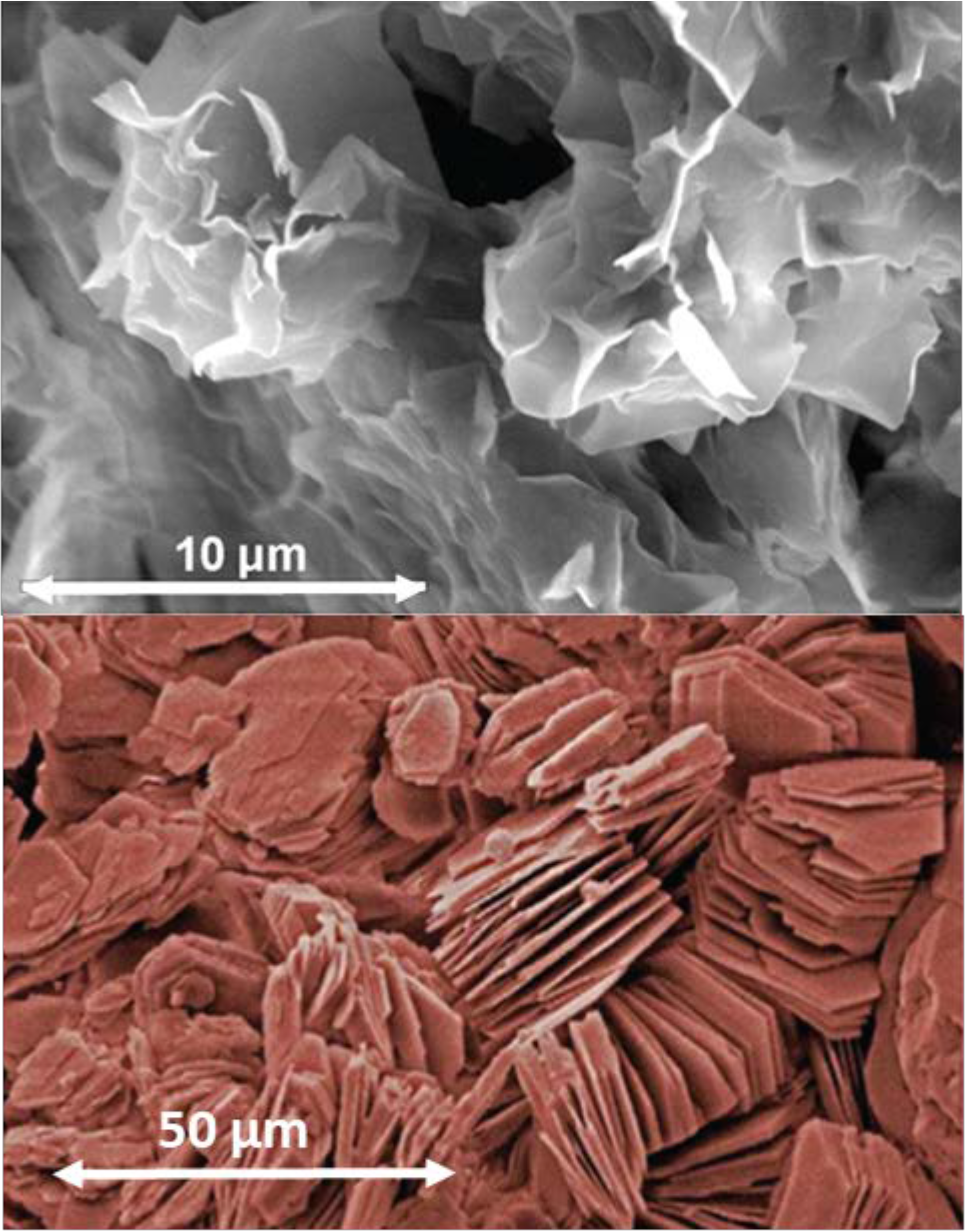
¶ C. Clay type and mineralogy
- Clay content is major factor determining CEC, but clay mineralogy has important role.
- Clay particles consist of sheets of atoms consisting of silicon, aluminum, potassium, etc.
- Arrangement of atomic sheets affects clay characteristics
- Chemical conditions present when clay was crystallizing determines amount of negative charge locked in particles
- Isomorphous substitution: cation replacement in minerals during mineral formation affects negative charges
- Clay particles consist of sheets of atoms consisting of silicon, aluminum, potassium, etc.
- Particles have both external and internal surfaces
- Internal layers are two planes of atoms.
- Layer on “bottom” side and layer on “top” side of each clay sheet.
- External surfaces are outside surfaces and edges of the clay sheet
- Both external and internal surfaces generally have negative charges and retain cations
- Internal layers are two planes of atoms.
- Clay minerals have different characteristics and different cation exchange capacities (see Table 1, Figures 1 and 3)
- Kaolinite; 1:1 non-swelling clay
- Vermiculite; 2:1 moderately swelling clay
- Can store or “fix” potassium ions in interlayer surfaces
- Illite, mica; 2:1 non-swelling clay
- Montmorillonite, smectite; 2:1 high swelling clay
- Chlorite; 2:1 non-swelling clay
¶ D. Organic matter, roots
- CEC of organic matter depends on soil conditions.
- Soil OM has CEC typically ranging from 150 to 400 mEq/100g.
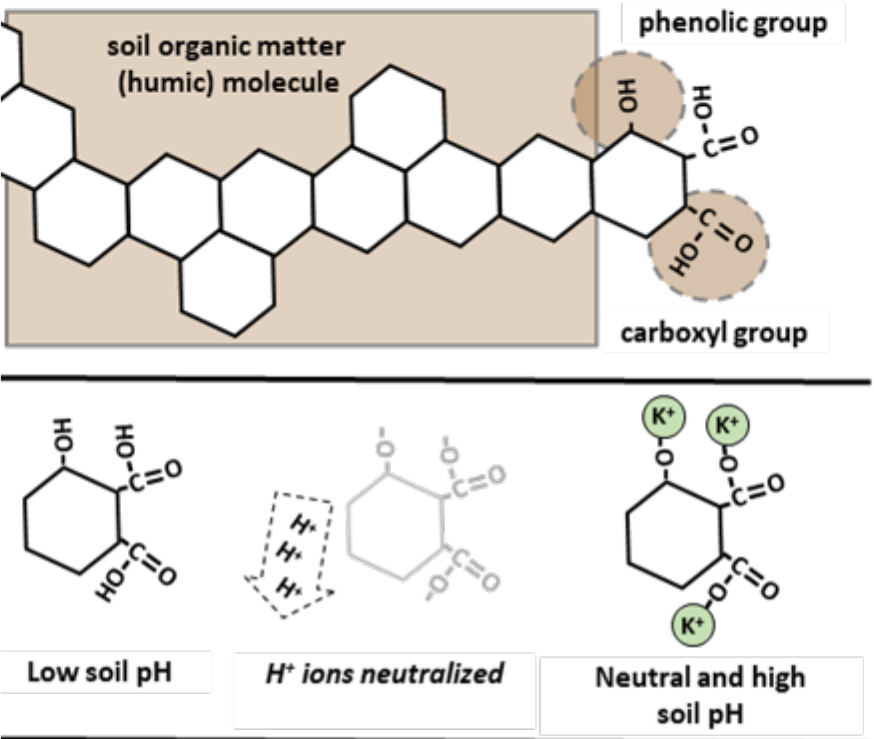
- OM has no permanent negative charge.
- During humification, lignin is chemically altered.
- Result is increase of phenolic and carboxyl groups in resulting humus molecules.
- Up to 90% of OM cation exchange sites due to these functional groups.
- Net negative charge is “pH dependent”.
- Hydrogen ions (H+) are stripped from hydroxyls (OH-) as soil pH increases.
- Hydroxyls are component of phenolic and carboxyl groups.
- Removing hydrogen ions leave negative charges (-O-) that can retain cations (see Figure 2)
- Organic matter CEC increases as pH increases
- At pH 2.5
- 1) CEC = 36 mEq/100g, 19% of CEC from SOM
- At pH 8.0
- CEC = 215 mEq/100g, 45% of CEC from SOM
- At pH 2.5
- Hydrogen ions (H+) are stripped from hydroxyls (OH-) as soil pH increases.
- Root surfaces have cation exchange sites.
- Root CEC mainly from carboxyl groups.
- Account for 70% to 90% of total root CEC.
- Monocotyledons ≈ 10 to 30 mEq/100g
- Monocots tend to absorb monovalent cations (e.g., K+) preferentially over divalent cations
- Dicotyledon roots ≈ 40 to 100 mEq/100g
- Dicots tend to absorb divalent cations (e.g., Ca2+) preferentially over monovalent cations
- Root CEC mainly from carboxyl groups.
¶ Figure 1. Range of Cation Exchange Capacity (CEC) Values
¶ Figure 2. Phenolic and Carboxylic Functional Groups
¶ E. Cation valence, hydrated radius
- Cations adsorbed onto colloids can be replaced by other, competing cations.
- Cations maintain state of equilibrium between solution and exchange surfaces.
- Cations on exchange complex (sum of exchange surface charges) reflect soil solution composition.
- Cation valence affects ability to exchange.
- Valence: Number representing ability of atom or group of atoms to combine with other atoms or groups of atoms
- Valence of common soil cations ranges from +1 to +3
- Highly charged cations (higher valence) tend to be held more tightly than cations with less charge (lower valence)
- Valence: Number representing ability of atom or group of atoms to combine with other atoms or groups of atoms
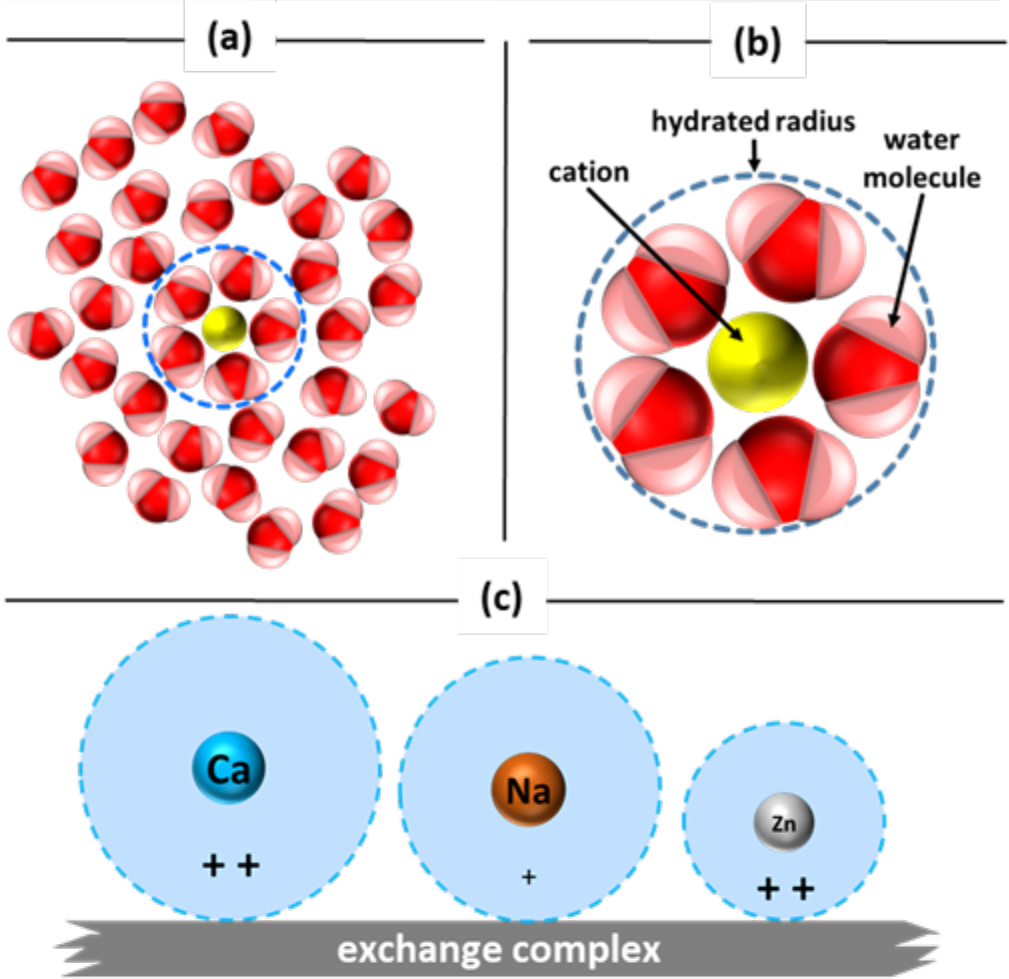
- Hydrated radius size affects ability to exchange
- Ions in water attract water molecules because of their charge (see Figure 3a)
- Ion become “hydrated” as whole water molecules bond to it forming spherical shell
- Hydrated radius: radius of actual ion and closely bound water molecules (see Figure 3b)
- Ionic radius: term used to describe size of ion
- Hydrated radius is larger than ionic radius, but size of hydrated radius varies between cations
- Cations with a small hydrated radius are bound more tightly to exchange complex
- Are less likely to be removed from exchange complex than cations with large radius
- Ions in water attract water molecules because of their charge (see Figure 3a)
- Monovalent cations are not necessarily easier to displace then divalent cations.
- Combined influence of cation charge and hydrated cation size affect equilibrium of exchange reactions. i. This influence can be summarized generally by “lysotropic series”.
- Relative strength of adsorption: Al3+ > H+ > Ca2+ > Mg2+ > K+ = NH4+ > Na+
- Adsorption strength decreases from left to right
- Cations with greater charge are more strongly adsorbed
- Hydrogen is unique because of size and charge density
- Smaller hydration radius size decreases distance between ion and exchange surface (see Figure 3c). i. Adsorption strength increases. ii. More tightly held cations are located closest to colloid surface
- Less likely to be leached away or be moved further downward through soil profile
¶ Figure 3. Water Molecules
¶ F. Concentration in soil solution
- Proportion and kinds of cations adsorbed on exchange sites is function of cation concentration in soil solution. a. Large quantity of one cation in solution can displace different cations from surface exchange sites
- If cation concentration in solution is high, there is increased chance or tendency for that cation to be adsorbed
- Soil-solution system equilibrium changes if soil solution composition changes
- System will adapt to develop new equilibrium between soil solution and exchange complex
- Altering equilibrium is management strategy used in soil remediation.
- Sodic soil: sodium ions occupy 15% or more of exchange sites.
- Solution Na+ ions supplied from irrigation water or very shallow ground water with elevated sodium content.
- Results in high proportion of adsorbed Na+ ions relative to adsorbed Ca2+ ions.
- Large amounts of gypsum (Ca2SO4) are applied to remedy sodic soil condition.
- Gypsum gradually dissolves in soil solution, providing source of Ca2+ ions.
- Results in high concentration of solution Ca2+ ions relative to solution Na+ ions.
- Solution Ca2+ ions begin to exchange with adsorbed Na+ ions to restore equilibrium between soil solution and soil exchange sites
- Displaced Na+ ions in solution are removed by leaching (“rinsing”) of soil solution with low sodium water
- Reduces concentration of Na+ ions relative to Ca2+ ions in soil solution
- Eventually results in establishing new equilibrium between soil solution and exchange complex. i. Soil gradually becomes non-sodic
- Excess gypsum or excess limestone may inflate summation CEC value beyond normal range for soil texture.
- Routine soil analysis with ammonium acetate or Mehlich extractant uses NH4+ ion to replace adsorbed cations in soil sample.
- Soil filtrate is analyzed to determine exchangeable cation concentrations.
- Extractant can dissolved solid minerals (lime, gypsum, etc.) from soil sample.
- Dissolved mineral cations in filtrate are included with exchangeable cations.
- Excess cation inflates calculated value for CEC by summation.
- AGVISE Laboratories experiment
- Applied gypsum at rates from 300, to 36,000 lb/ac.
- Essentially no change in K, Mg, or Na concentrations.
- Calcium concentration increased from 2460 ppm Ca to 6000 ppm Ca
- No change in soil texture or number of exchange sites
- Resulted in summation CEC increasing from 17 mEq/100g (0 gypsum) to 35 mEq/100g (36,000 lb/ac)
¶ G. Soil pH
- CEC provided by OM entirely determined by soil pH.
- OM loses ability to hold cations as soil becomes more acidic.
- Clay has pH dependent charge, but to lesser degree.
- About 5% to 10% of negative charge for 2:1 clays depends on pH
- For kaolinite clays, 50% or more of the CEC can be pH dependent
¶ H. Anion Exchange Capacity (AEC)
- Majority of the world’s soils have cation exchange capacity.
- Less weathered soils under neutral and alkaline conditions have CEC with small amount of AEC, i. Example, at pH 8.2:
- AEC of montmorillonite ≈ 1 mEq/100g, where CEC ≈ 118 mEq/100g.
- AEC of kaolinite clay ≈ 2 mEq/100g, where CEC ≈ 4 mEq/100g.
- Highly weathered, tropical soils have CEC, but also have anion exchange capacity (AEC).
- 1:1 clays more prone to generate AEC than 2:1 clays.
- Less weathered soils under neutral and alkaline conditions have CEC with small amount of AEC, i. Example, at pH 8.2:
- Highly weathered soil generates AEC under acidic conditions.
- Soil becomes positively charged.
- Hydroxyls take on hydrogen ions or become protonated.
- AEC attracts, retains, and supplies negatively charged anions.
- e.g., sulfate (SO42-), phosphate (HPO42- or H2PO4-), nitrate (NO3-), chloride (Cl-)
- Cations are leached from soils with high AEC
- e.g., calcium (Ca2+), magnesium (Mg2+), ammonium (NH4+), potassium (K+)
- Fertility management needed to prevent cation nutrient deficiencies
- Soil becomes positively charged.
- Mineral types affect pH at which soils develop AEC
- Highly weathered kaolinite (1:1 clay)
- AEC may be as high as 43 mEq/100g at pH 4.7
- Aluminum and iron oxides
- Allophanes and imogolites of volcanic soils
- Highly weathered kaolinite (1:1 clay)
- Organic matter only generates AEC at very low pH
- Still good source of CEC
- Certain soils all have AEC under acidic conditions
- Highly weathered Ultisols and Oxisols
- Volcanic Andisols
- Organic Histosols
¶ Figure 4. Clay Particle Types
1(Refer to Crop File 1.05.100 “Cation-Anion Exchange” and 4.01.010 “Cation Exchange Capacity (CEC) basics” for background information related to this Crop File.)
21 micron = 0.001 millimeter ≈ 1/25,000th inch
¶ References
Tisdale, S.L., et. al. 1993. "Basic Soil-Plant Relationships" in Soil Fertility and Fertilizers. MacMillan Pub. Co., New York. pp. 80-94.
Mickkelsen, R. 2011. Cation Exchange: A Review. Insights, Nov. 2011. Intl Plant Nutrition Institute, Merced CA. 3 pg.
McClellan, T. Soil Nutrient Management for Maui County: Cation exchange. College of Tropical Agriculture and Human Resources. Univ. of Hawaii. http://www.ctahr.hawaii.edu/mauisoil/c_relationship.aspx.Aaccessed 14 June 2016
Sparks, D.L. 2003. Chemistry of Soil Organic Matter in Environmental Soil Chemistry (Second Edition). Pg. 75-113
Foth, H.D. 1984. “Soil Chemistry” in Fundamentals of Soil Science, 7th ed. John Wiley & Sons, New York. pp. 191-197
Grain SA . Soil: The Producer’s Most Important Asset, Part 4: The clay minerals. Grain SA, Pretoria South Africa. https://www.grainsa.co.za/soil-the-producers-most-important-asset-part-4-the-clay-minerals. Accessed 08 Dec 2020.
AGVISE Laboratories. Gypsum Magic: Part 3. Changing Cation Ratios, Soil pH and CEC. https://www.agvise.com/tech_art/gypsum3.php. Accessed 28 Mar 2008