⇦ Back to Soil Resource Management Home
¶ Introduction
Cation exchange capacity (CEC) is an important soil property. It is a good indicator of the “surface area” available to retain and release certain nutrients.[1]
¶ A. Basic cation exchange concepts
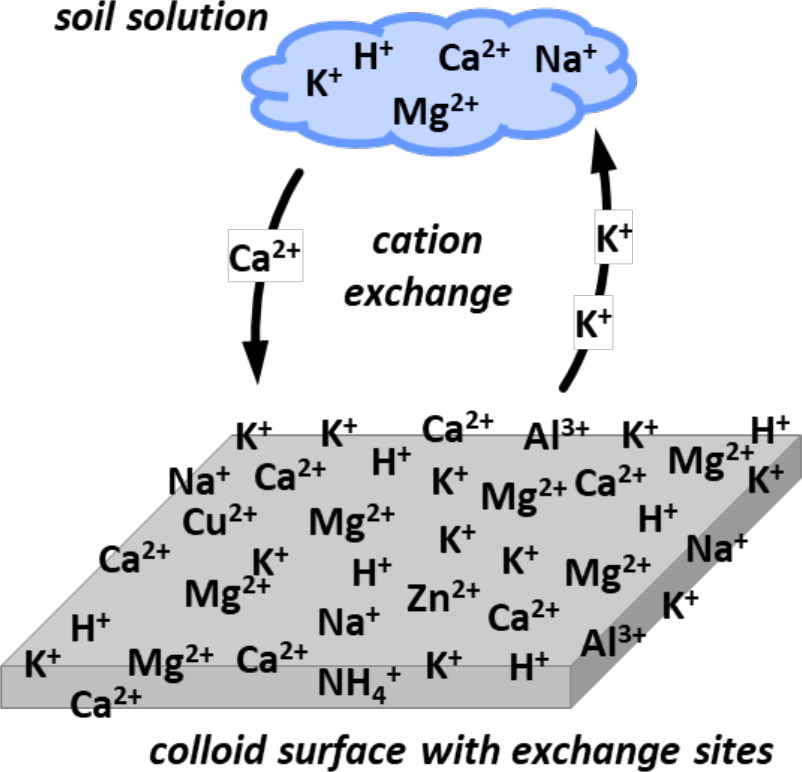
- Cations in solution can exchange with cations adsorbed onto surface of soil colloids.
- Elements and molecules in soil have positive charge (cations) or negative charge (anions).
- Cation charges typically range from +1 to +3.
- Cation exchange sites: negatively charged sites on clay colloids and humic colloids.
- Cations are retained at these sites by electrostatic forces
- Cations adsorbed onto colloids can be replaced by other, competing cations
- Large quantity of one cation can displace different cations from surface exchange sites.
- Elements and molecules in soil have positive charge (cations) or negative charge (anions).
- Important for retaining inorganic and organic cations.
- Major source of plant available K+, Mg2+, Ca2+.
- Adsorbs many trace metals.
- Zn2+, Cu2+, Cd2+, Pb2+, Ni2+, others.
- Adsorbs pesticides, other organic compounds with strong positive charge potentially affecting application rate.
- e.g., glyphosate (Roundup®), paraquat
- Affects soil “buffering capacity”.
- Moderates change in solution pH and nutrient concentrations
- e.g., high CEC requires high limestone rates to increase pH of acid soils.
- Moderates change in solution pH and nutrient concentrations
- Exchange reactions are reversible, rapid, and “balanced” with respect to charge
- Positive charges of cations adsorbed on exchange surfaces are in quantities equivalent to number of negative surface charges
- Cation charges typically range from +1 to +3
- Cations are held on exchange surfaces until replaced (exchanged) with other cations in soil solution
- Ease of cation displacement from exchange surface is a function of cation size and charge
- Only small percentage of cations will be dissolved in soil water (in soil solution) at any given time
- Solution cations are immediately available for plant uptake from solution
- Positive charges of cations adsorbed on exchange surfaces are in quantities equivalent to number of negative surface charges
- Think of soil solution as sea that is full of cations.
- Cations are waiting to anchor in any empty harbor (any surface exchange site or sites).
- High CEC soil has more harbors; low CEC soil has fewer.
- When one cation leaves harbor, another cation from surrounding sea (soil solution) takes its place.
- Number of negative surface charges are equal to number of positive adsorbed-cation charges.
- Bigger cations (more positive charge) may shove several smaller cations out of harbor.
- Charged cation from solution will displace equal number of adsorbed cation charges during exchange reactions.
- Example exchange: 2 K+-{soil} with 1 Ca2+ in solution → 2 K+ in solution with 1 Ca2+-{soil}
- Cations are waiting to anchor in any empty harbor (any surface exchange site or sites).
¶ Figure 1. One Divalent Cation Exchanges With Two Monovalent Cations
¶ B. Measuring CEC
- CEC is measure of number of sites on soil colloid surfaces available to adsorb and release cations.
- Expressed as total number of surface charges per unit of soil
- Milliequivalents per 100 grams of soil (mEq/100g)
- Unit commonly used by soil testing laboratories
- Centimoles of charge per kilogram of soil (cmol/kg)
- Metric unit listed in International System.
- 1 cmol/kg = 1 meq/100g
- Number of cations that can be retained on exchange surface depends on particular charge of that cation
- Soil with 1 cmol/kg of charge can retain 1 millequivalent weight of cation per 100 grams.
- CEC = 1 mEq/100g ≈ 600 quintillion (600,000,000,000,000,000,000) adsorption sites in 100 grams of soil.
- 100 grams is about 8 tablespoons of soil
- CEC = 1 mEq/100g, exchange sites could be filled with 1.0 mg of monovalent cation (e.g., H+, K+, Na+NH4+)
- Sites could be filled with 0.5 mg of divalent cation (e.g., Ca2+, Mg2+)
- Sites could be filled with 0.33 mg of trivalent cation (e.g., Al3+)
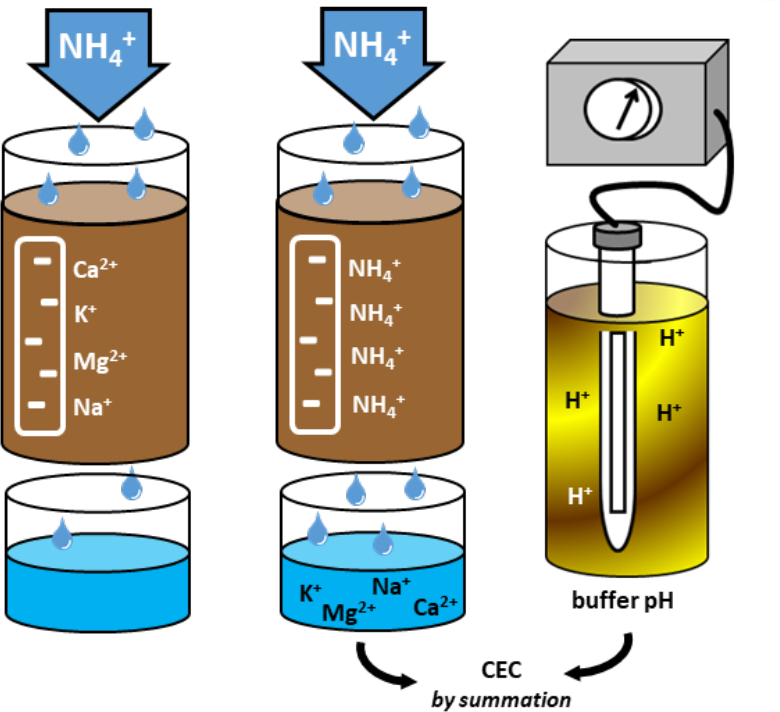
- CEC determined by treating soil sample with excess solution containing single cation.
- Routine soil test uses ammonium acetate solution or Mehlich extractant (see Figure 2)
- NH4+ ions in extracting solution replace cations on soil exchange sites. ii. Soil is filtered and discarded
- Filtered extracting solution is analyzed for exchangeable cations (K+, Ca2+, Mg2+, and Na+) used in fertility management
- Results are used to calculate “CEC by summation”.
- EPA method 9081
- More accurate, especially in calcareous soils, but more time-consuming and expensive
- Soil sample is first treated with sodium acetate solution, then rinsed with alcohol
- Na+ ions displace exchangeable cations
- . Sample is then treated with ammonium acetate, then filtered
- NH4+ ions displace exchangeable Na+ ions. iv. Filtrate is analyzed for sodium.
- Routine soil test uses ammonium acetate solution or Mehlich extractant (see Figure 2)
¶ Figure 2. Determining Estimated CEC
¶ C. Calculating estimated “CEC by summation”
- Estimated CEC (as mEq/100g) = sum of exchangeable cations plus exchangeable acidity.
- Determined from results of routine soil analysis.
- mEq of exchangeable cation = soil analysis result (as ppm) / equivalent weight of cation i. K mEq/100g = ppm K ÷ 390 ii.Ca mEq/100g = ppm Ca ÷ 200 iii. Mg mEq/100g = ppm Mg ÷ 120 iv. Na mEq/100g = ppm Na ÷ 230
- mEq of exchangeable acidity (H+) estimated from buffer pH result
- H mEq/100 g = (7 – buffer pH) x 12
- Calculate sum of components for estimated CEC value (see Table 1 for example calculations)
- May overestimate CEC in calcareous or gypsic soils.
- Ammonium acetate solution (pH 7) and Mehlich-3 solution (pH 2.5) may dissolve free lime or gypsum in sample.
- May inflate calcium result because dissolved calcium reported as exchangeable calcium
- Recommend capping calcium result at 5000 ppm Ca for calculation purposes
- Ammonium acetate solution (pH 7) and Mehlich-3 solution (pH 2.5) may dissolve free lime or gypsum in sample.
¶ Table 1. Example Calculations, CEC by Summation |
|||
| Soil #1 test result | Equivalent weights | mEq/100g | |
| pH | 5.0 | --- | --- |
| K ppm | 192 | ÷ 390 = | 0.5 |
| Ca ppm | 2560 | ÷ 200 = | 12.8 |
| Mg ppm | 452 | ÷ 120 = | 3.8 |
| Na ppm | 46 | ÷ 230 = | 0.2 |
| BpH | 5.7 | (7 – BpH) x 12 = | 3.6 |
| CEC by summation = | 20.9 | ||
| Soil #2 test result | Equivalent weights | mEq/100g | |
| pH | 7.7 | --- | --- |
| K ppm | 709 | ÷ 390 = | 1.8 |
| Ca ppm | |||
| 5000 | ÷ 200 = | 25 | |
| Mg ppm | 327 | ÷ 120 = | 2.7 |
| Na ppm | 10 | ÷ 230 = | 0.0 |
| BpH | 0 | (7 – BpH) x 12 = | 0.0 |
| CEC by summation = | 29.5 | ||
| *Note: Ca result capped at 5000 for calculation | |||
[1] Refer to Crop File 1.05.100 “Cation-Anion Exchange” for background information.