⇦ Back to Fertilizer Lime Amendment Technology and Use Home
¶ Introduction
This Crop File provides background information for interpreting results of other Crop Files having “Typical analysis” ranges for various types of livestock manure. The individual Crop File contents were obtained from survey results. Results for each of these Crop Files were grouped by livestock type (beef, dairy, swine, poultry) and by percentage of total solids.
¶ A. Manure Survey Background
- Manure properties are inconsistent and heterogenous
- Lab analysis results for many manure samples were downloaded to identify practical range of chemical and physical properties
- Includes manure and livestock waste samples received from 2008 to 2020
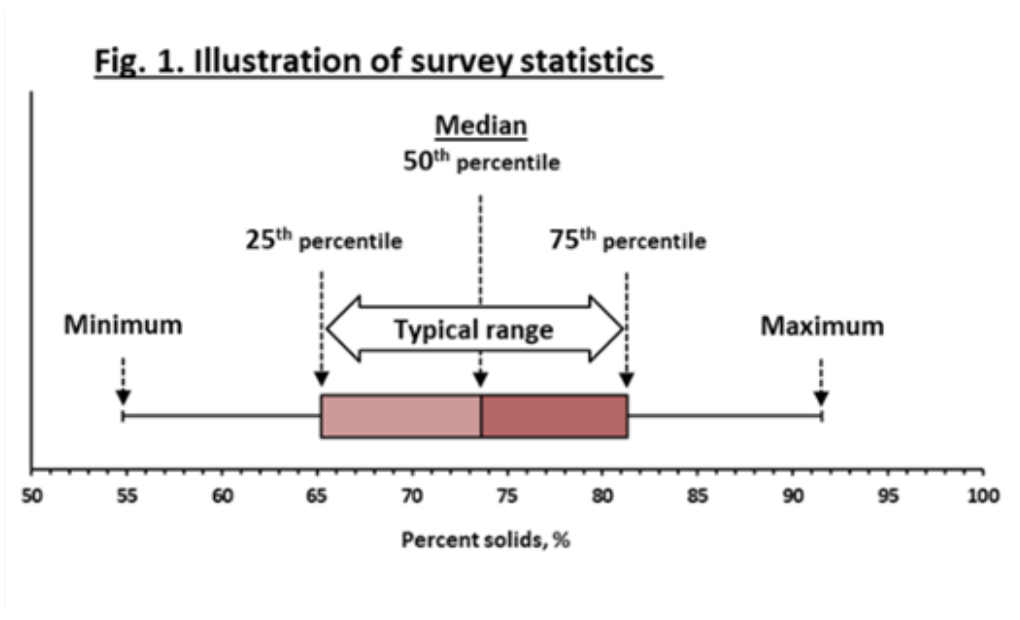
- Survey results expressed as “median” and “typical range” values (illustrated in Figure 1)
- Median value
- Is value of 50th percentile
- One-half (50%) of results less than median value
- One-half (50%) of results greater than median
- Typical range
- Represents values of 25th, 50th and 75th percentiles
- “Typical range” includes middle half (50%) of sample results
- One-fourth of sample results below typical range
- One-fourth of sample results above typical range
- Median value
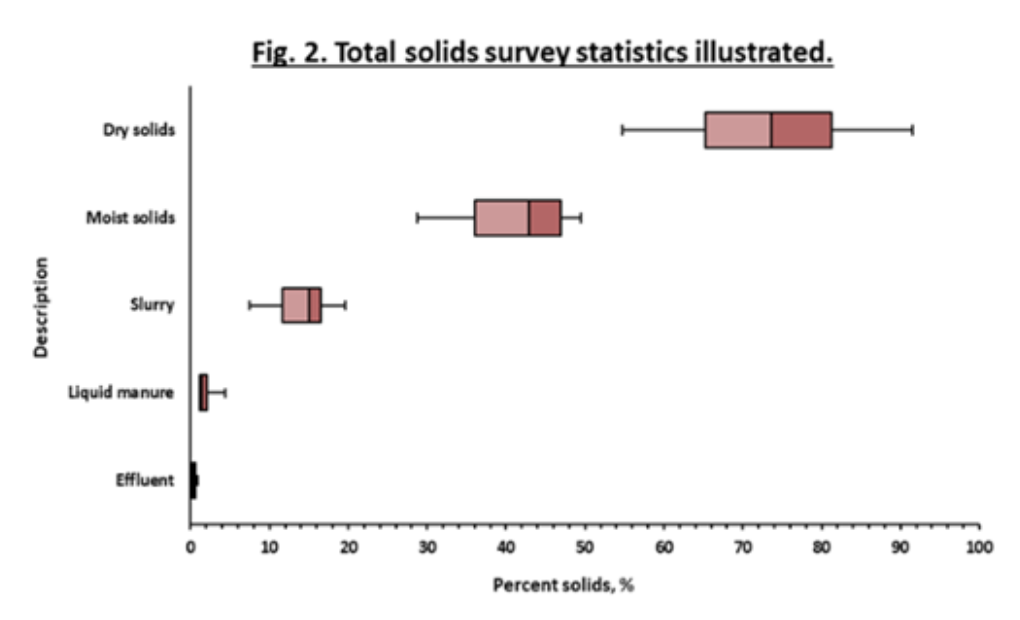
- Survey results divided into five categories using percent solids
- Manure category assigned according to solids content. Examples in Figure 3
- “Effluent” = 1% solids or less
- “Liquid manure” = 1% to 5% solids
- “Slurry” = 5% to 25% solids
- “Moist solid manure” = 25% to 50% solids
- “Dry solid manure” = 50% solids or greater
- Figure 2 illustrates potential differences that may be seen in range of total solids within manure categories
- Manure category assigned according to solids content. Examples in Figure 3
¶ Figure 1. Illustration of Survey Statistics
¶ Figure 2. Total Solids Survey Statistics Illustrated
¶ B. NRCS Definitions
- Manure
- Combinations of feces and urine only
- Waste
- Includes manure plus other material
- May include bedding, soil, wasted feed, wasted drinking water.
- May include water used for sanitary and flushing purposes
- Liquid waste or liquid manure
- Total solids content: 5% or less
- Moisture content: 95% or more
- Has qualities very much like water
- Semi-liquid (slurry) or semi-solid
- Total solids content: between about 5% and 25%
- Moisture content: between about 75% and 95% percent
- Solid manure or solid waste
- Total solids content: about 25% or more.
- Moisture content: about 75% or less
- Exhibits properties of a solid
- Can be stacked and hold definite angle of repose
¶ Figure 3. Approximate Manure Consistency with Differing Solids Content

¶ C. Terminology
- Major nutrients
- Are essential plant nutrients typically required in largest amounts
- Includes nitrogen (N), phosphorus (P), potassium (K)
- N content expressed as “total nitrogen” and as three different nitrogen fractions (see below)
- P and K expressed as fertilizer equivalents: “phosphate, (P2O5)” and “potash (K2O)”
- Secondary major nutrients
- Are essential plant nutrients required in smaller, but significant, amounts.
- Includes calcium (Ca), magnesium (Mg), and sulfur (S)
- Sodium (Na) not considered essential nutrient, but can contribute to salinity
- Micronutrients
- Are essential plant nutrients required in very small (“micro”) amounts.
- Includes zinc (Zn), iron (Fe), manganese (Mn), copper (Cu), and boron (B)
- Total nitrogen, N
- Sum of ammoniacal-nitrogen, organic nitrogen, and nitrate-nitrogen
- Organic nitrogen
- Total nitrogen minus ammoniacal-nitrogen and nitrate-nitrogen
- Can be considered “non-mineral” nitrogen
- Ammoniacal-nitrogen
- Includes both ammonia (NH3) and ammonium (NH4+) nitrogen compounds
- Ammonia-nitrogen (NH3-N)
- Gaseous form of ammoniacal nitrogen
- Is dissolved in moisture fraction of manure
- Nonionized form of ammoniacal-nitrogen (NH3)
- Can be lost by volatilization
- Can be ionized to ammonium
- Is potentially toxic to microbes and plants
- Gaseous form of ammoniacal nitrogen
- Ammonium-nitrogen (NH4–N)
- Positively ionized form of ammoniacal nitrogen (NH4+)
- Considered immobile; can become attached to soil clays
- Can be utilized by plants or microbes
- Positively ionized form of ammoniacal nitrogen (NH4+)
- Nitrate-nitrogen (NO3–N)
- Negatively ionized form of nitrogen (NO3-)
- Can be lost by denitrification, percolation (leaching), surface runoff ii. Can be utilized by plants or microbes
- Negatively ionized form of nitrogen (NO3-)
- mg/L (milligrams per liter
- Expresses concentration of substance in liquid sample
- 1 mg/L = one part of target substance in one million parts of total liquid sample
- mg/kg (milligram per kilogram)
- Expresses concentration of substance in solid sample
- 1 mg/kg = one part of target substance in one million parts of total solid sample
- ppm (parts per million)
- Expresses concentration of substance in total sample.
- 1 ppm = one part of target substance in one million parts of total sample c. ppm ≈ mg/kg ≈ mg/L
- Conversion factors and equivalents
- 1% = 10,000 ppm
- lb/ton = % * 20
- lb/ton = ppm * 0.0020
- lb/1000-gal = % * 83
- lb/1000-gal = ppm * 0.0083
- lb/1000-gal = mg/L * 0.0083
- % P2O5 = % P * 2.28
- % K2O = % K * 1.16
- 1 gallon of water = 8.345 pounds
- 1,000 µmho/cm = 1 mmho/cm
- Moisture %
- That part of sample removed by evaporation and oven dried at 217 °F (103 °C).
- Moisture % + solids % = 100 %
- Solids %
- Also known as “dry matter %” or “total solids %”
- Residue remaining after moisture is removed from sample by evaporation
- Solids % + moisture % = 100 %
- Organic matter %
- Also known as “volatile solids %”
- That part of total solids driven off as volatile (or combustible) gases when heated to 1112 °F (600 °C)
- Includes various carbon-containing materials; considered “non-mineral” portion of solids
- May include undigested feedstuffs, bedding, waste feed, etc
- Organic matter_% = solids_% - ash_%
- Estimated organic carbon, %C
- Calculated from organic matter content (e.g., volatile solids)
- %_C ≈ % OM x 0.58
- Ash %
- Also known as “fixed solids %”.
- That part of total solids remaining after volatile gases driven off at 1112 °F (600 °C)
- Includes soil (sand, clay) and other mineral matter
- Ash % + organic matter % = solids %
- Carbon-to-nitrogen ratio, C:N
- Ratio of mass of carbon to mass of nitrogen
- C:N = 10:1 = ten parts carbon to one part nitrogen
- Has effect on decomposition of organic materials (e.g., crop residues, bedding, etc.)
- Materials with C:N ratio less than 25:1 to 35:1 are more readily decomposed (“mineralized”) when soil incorporated
- Materials with C:N ratio greater than 30:1 to 40:1 can cause temporary soil nitrogen deficit (“immobilization”) when soil incorporated
- Carbon content estimated by using ash content
- Carbon % ≈ (total solids % – ash %) / 1.72
- Ratio of mass of carbon to mass of nitrogen
- Estimated first year mineralization
- Estimation of plant available nitrogen to be mineralized from organic nitrogen fraction of manure during first growing season after application
- i. . Additonal nitrogen may be mineralized in second and successive growing seasons, depending on field conditions
- Calculated from organic nitrogen concentration
- % mineralized-N ≈ 60 - (1.5 * C:N)
- Estimation of plant available nitrogen to be mineralized from organic nitrogen fraction of manure during first growing season after application
- Electrical conductivity
- Standard measure of soil salinity expressed as “mmho/cm” or “(millimhos per centimeter)”
- High soil salinity interferes with plant’s ability to absorb soil water and exchange plant nutrients
- Reduces plant growth and seed germination
- Limits the choice of crops that can be successfully grown
- May be expressed as “µmho/cm” or “micromhos per centimeter”
- 1,000 µmho/cm = 1 mmho/cm
- Total salts, estimated ppm
- Calculated value based on electrical conductivity result.
- Total salts, estimated ppm = EC mmho/cm x 700)
- Sodium adsorption ratio, SAR
- Calculation of sodium concentration (mg Na/L) relative to concentrations of calcium (mg Ca/L) and magnesium (Mg mg/L) in liquid sample
- SAR value of about 5 to 7 or greater indicates potential for long-term application of waste material to degrade soil structure, reduce permeability, and restrict water intake. Depends on soil texture and other characteristics
- CROSS (Cation Ratio of Structural Stability)
- Revision of SAR calculation to include potential additional impact of potassium and magnesium on soil structure
¶ References
USDA-SCS. 1992. Agricultural Waste Management Field Handbook, Part 651, USDA-Soil Conservation Service, Chap. 11: "Waste Utilization".
Vigil, M. & D. Kissel. 1991. Equations for estimated the amount of nitrogen mineralized from crop residues. Soil Sci. Soc. Amer. Proc. 55:757-761.
Powers, W.L., et. al. 1975. Guidelines for Land Disposal of Feedlot Lagoon Water, Pub. C-485.
Coop. Ext. Serv., Kansas State Univ., Manhattan KS. 7 pg.
Powers, W.L., et. al. 1974.Guidelines for Applying Beef Feedlot Manure to Fields, Pub. C-502. Coop. Ext. Serv., Kansas State Univ., Manhattan KS. 11 pg.
Oster, Sposito, & Smith. 2016. Accounting for potassium and magnesium in irrigation water quality assessment. California Agriculture 70:71-76. Univ. of California Coop. Ext., Davis CA.