⇦ Back to Fertilizer Lime Amendment Technology and Use Home
¶ Introduction
The term "micronutrient chelate" is used to describe a class of micronutrient fertilizer materials. There are two types of chelated fertilizers: synthetic chelates and natural organic complexes. They are usually formulated as liquids, but are sometimes sold as dry powders.
¶ Definition
A “chelate” is defined as "a cyclic molecular structure, usually containing five or six atoms in a ring in which a central metallic ion is held in a coordination complex". More simply, a chelate is a sort of "molecular cage" that protects a metallic ion from unwanted chemical reactions.
Chelates are common compounds found in nature. For example, hemoglobin is a chelate found in the human blood stream. It surrounds and protects an oxygen molecule and allow it to be transported from the lungs, through the blood stream, and into the appropriate body cells.
¶ The Chelating Process
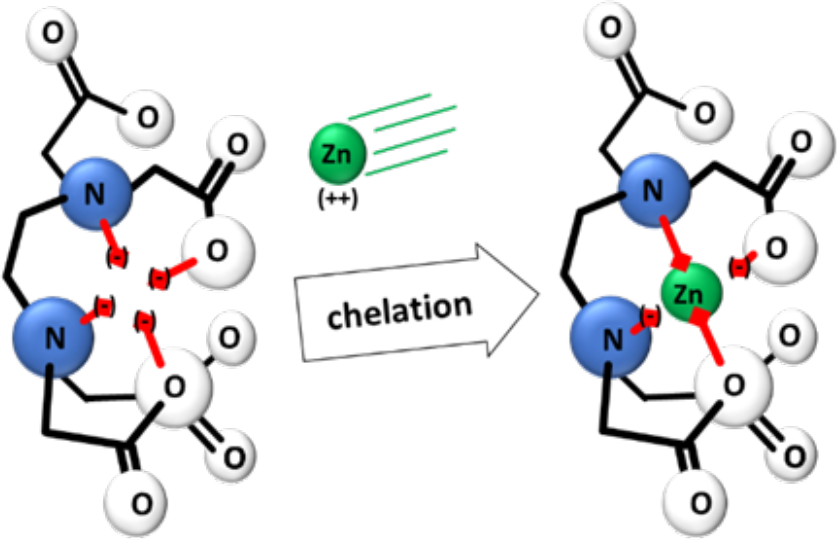
The word chelate comes from the Greek word "chela", meaning “claw”. Figure 1 shows an example of a chelate and the chelating process.
A chelate molecule (EDTA, for example) may have four negatively charged "arms" and does resemble a claw. Metallic ions like zinc, iron, manganese, or copper have two positive charges. Two of the chelate arms form a bond with the metallic ion during the chelation process. This forms a ring-like molecular structure around the ion. Chelation protects the micronutrient cation by making it difficult for it to react with any other molecules.
The two negatively charged arms cancel out the two positive charges of the metallic ion. Since two of the chelate arms remains unbonded, the newly formed, chelate-metallic ion molecule has an overall negative charge. For a time, it acts like an anion in the soil environment with mobility similar to nitrate or chloride. At some point, the structure (i.e., “molecular cage”) releases the micronutrient cation where it becomes available for plant uptake or for interaction with other compounds in the soil.
¶ Chelated Micronutrient Fertilizers
Chelated micronutrient fertilizers can be classified as either synthetic chelates or as organic complexes. There are five synthetic materials commonly used to chelate micronutrients:
- EDTA - ethylenediaminetetraacetic acid
- HEDTA - hydroxyethylethylenediaminetriacetic acid
- DTPA - diethylenetriaminepentaacetic acid
- EDDHA - ethylenediamine di(o-hydroxy- phenylacetic) acid
- NTA - nitrilotriacetic acid
Organic complexes are manufactured from organic materials that have chelating-type properties, but they are not “true” chelates. These materials contain organic molecules that are able to complex or bind metallic micronutrient cations. These complexing materials include lignosulfonates, phenols, and polyflavinoids, often obtained from byproducts of the wood and paper pulp industry. Sucrose-type materials, like cane sugar molasses, are used in some sucrate products.
¶ Figure 1. Illustration of Chelation Process With EDTA (C10H16N2O8)
¶ Figure 2. Chemical Structures of Common Fertilizer Chelates

¶ How Chelates Behave in the Soil
Metallic cations, (like zinc, iron, or copper) are considered immobile nutrients. Zinc ions, for example, only move about ¼ to ⅓ inch from the point of application in a limed soil and about ⅓ to ½ inch in an unlimed soil.
Cations are positively charged and so attach rapidly to the negatively charged surfaces of soil clay and organic matter particles. They can also form insoluble compounds by chemically combining with carbonates, phosphates, and other materials in the soil solution.
The chelate bond allows a positively charged metallic cation to behave as an anion (negatively charged ion). Chelate/metal anions are not attracted to the surfaces of soil particles. The chelate/metal anion remains mobile in the soil solution and does not form insoluble compounds, like carbonates or phosphates. It can migrate through the soil with moisture movement toward the plant root surface, where the metallic cation can be potentially available for uptake.
The chelate bond will eventually release the metallic cation into the soil solution where the cation may again become immobilized. The rate of chelate release depends on the particular chelating material, soil chemistry (especially pH), and the soil environment.
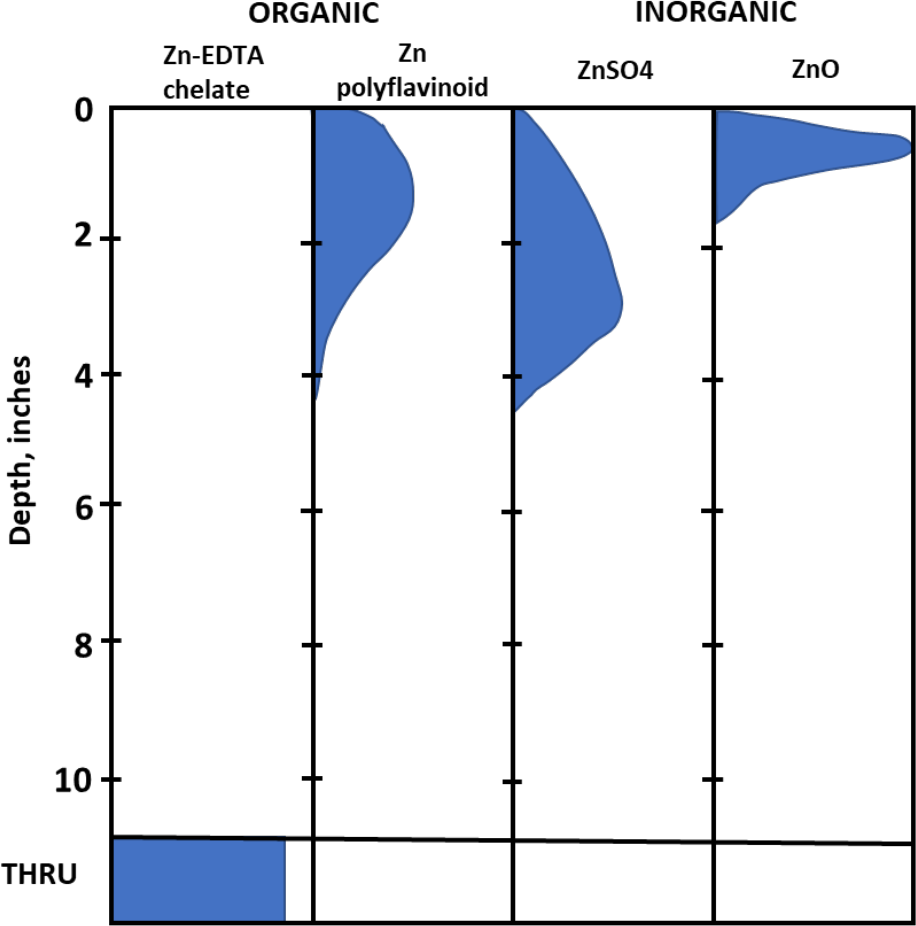
Chelate mobility is demonstrated by the research results shown in Figure 3. Four zinc materials were placed on the surface of a loamy fine sand, then leached with 20 inches of water.
Zinc oxide is insoluble in water so moved only about 1½ inches through the column. The zinc in the polyflavinoid material reverted to a metallic cation, attached to the negatively charged soil particles, and leached only about 2 to 2½ inches below the surface.
The zinc in the zinc sulfate was able to migrate farther through the profile, 3 to 4 inches, but also became attached to the soil clay exchange surfaces. The negatively charged zinc-EDTA chelate did not attach to the soil particles and was leached completely out of the column.
¶ Figure 3. Distribution of Added Zinc