⇦ Back to Fertilizer Lime Amendment Technology and Use Home
¶ Introduction
Thiosulfate (S2O32-) fertilizers are clear liquids that provide a source of sulfur (S) and can be used in a variety of situations. They also contain other nutrients including nitrogen (N) as ammonium (ATS), potassium (KTS), calcium (CaTS), ormagnesium (MgTS).
¶ Production
ATS is the most commonly used sulfur-containing fluid fertilizer. It is made by reaction of sulfur dioxide, elemental sulfur, and aqueous ammonia. Other common fluid thiosulfate fertilizers are produced similarly.
Thiosulfates are highly soluble in water and are compatible with many other fluid fertilizers. ATS is often mixed with urea ammonium nitrate (UAN) to produce a fertilizer with the analysis 28-0-0-5%S.
¶ Table 1. |
||||
| Abbreviation: | ATS | KTS | CaTS | MgTS |
| Chemical formula | (NH4)2S2O3 | K2S2O3 | CaS2O3 | MgS2O3 |
| Fertilizer analysis: | ||||
| Sulfur | 25% | 17% | 10% | 10% |
| Other | 21% N | 25% K2O | 6% Ca | 4% Mg |
| Density, lb/gal | 11.2 | 12.2 | 10.4 | 10.3 |
| Solution pH | 7.0 to 8.5 | 7.5 to 8.0 | 6.5 to 8.0 | 6.5 to 7.5 |
¶ Agricultural Use
After application to soil, most of the thiosulfate quickly reacts to form tetrathionate, which is subsequently converted to sulfate. Thiosulfate is not generally available for plant uptake until it is converted to sulfate. In warm soils, this process is largely complete within one to two weeks.
Thiosulfate is a chemical “reducing agent” and it also produces acidity after oxidation of the S. Due to these properties, thiosulfate molecules have unique effects on soil chemistry and biology. For example, a band application of ATS has been shown to improve the solubility of some micronutrients. Local guidelines should be followed for maximum rates for placement in the seed row.
¶ Figure 1. Thiosulfate Forms
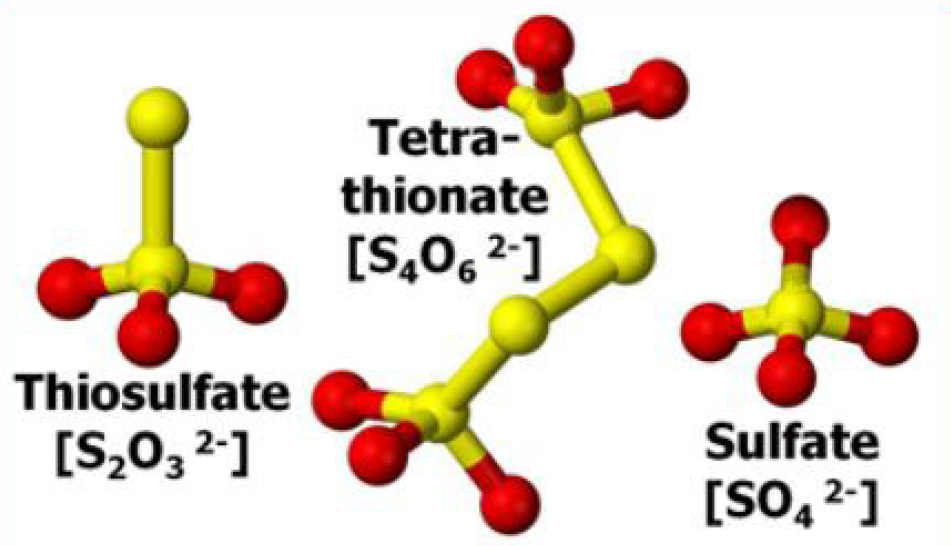
This inhibiting effect is likely due to the formation and presence of the intermediate tetrathionate, rather than the thiosulfate itself.
Thiosulfate can slow the rate of urea hydrolysis - the conversion of urea to ammonium (NH4+) - and reduce loss of ammonia (NH3) as a gas when ATS is mixed with UAN. This inhibiting effect is likely due to the formation and presence of the intermediate tetrathionate, rather than the thiosulfate itself.
Nitrification...the conversion of ammonium to nitrate is also slowed in the presence of ATS. Although the initial pH of thiosulfate fertilizers is near neutral, thiosulfate oxidizes to form sulfuric acid and the ammonium forms nitric acid, thus resulting in slight soil acidification in the application zone.
Thiosulfates may be applied through surface and overhead irrigation systems, sprinklers, and drip irrigation. Many of them are used in foliar sprays toprovide a rapid source of plant nutrition (not recommended with ammonium thiosulfate).
¶ Management Practices
Thiosulfates are valuable fertilizer materials because they are easy to handle and apply, require minimal safety precautions, and are compatible with many other common fertilizers.
These fertilizers should not be mixed with highly acidic solutions since this will cause the decomposition of the thiosulfate molecule and subsequent release of harmful sulfur dioxide gas.
¶ Non-agricultural Use
Thiosulfate materials are used in a variety of industrial applications. In photographic processing, they are used to bind silver atoms present in film or paper. Sodium thiosulfate is used in water treatment systems to remove chlorine. It is also used for gold extraction, since it forms a strong complex with this metal in a non-toxic process.
¶ References
IPNI. Nutrient Source Specifics No. 18: Thiosulfate. International Plant Nutrition Institute, Norcross Georgia. 1 pg.