⇦ Back to Fertilizer Lime Amendment Technology and Use Home
¶ Introduction
This Crop File discusses some basic characteristics and manufacture of common commercial phosphate fertilizers.
¶ A. History of Phosphorus Fertilizer
- Before 1940’s and 1950’s
- Early sources were commonly animal-based
- Typically had low phosphorus content
- Examples:
- Livestock manure
- Bone meal
- Guano (bat manure)
- Ground rock phosphate
- Commercial fertilizer production very limited
- “Normal superphosphate” or “single superphosphate” (0-20-0-12% sulfur)
- Produced by treating animal bones or rock phosphate with acid
- Some nitric phosphates
- Materials were powdery and dusty
- “Normal superphosphate” or “single superphosphate” (0-20-0-12% sulfur)
- Early sources were commonly animal-based
- After 1940’s
- Tennessee Valley Authority (TVA) National Fertilizer Development Center introduced many current phosphate fertilizer production technologies
- Commercial-scale “continuous rotary drum ammoniator-granulator” process to produce granular fertilizers demonstrated in 1952
- Process to produce diammonium phosphate (DAP, 18-46-0) introduced in 1955
- Wet process phosphoric acid procedure developed in 1964
- T-pipe reactor used to produce liquid ammonium polyphosphate invented in 1972
- Tennessee Valley Authority (TVA) National Fertilizer Development Center introduced many current phosphate fertilizer production technologies
¶ B. Phosphate Rock
- Definition: unprocessed ore and processed concentrates containing some form of apatite
- Apatite: group of calcium phosphate minerals
- Formed by deposition of phosphate-rich materials in prehistoric sea bottoms
- May found in sedimentary or igneous rocks
- 80% of world production from sedimentary deposits
- Primarily located in China, the Middle East, northern Africa, and United States
- Igneous deposits are associated with carbonatites and silica-deficient intrusions.
- Are mined in Brazil, Canada, Finland, Russia, South Africa, and Zimbabwe
- 80% of world production from sedimentary deposits
¶ C. Phosphate rock production
- China produces about 40% of world production
- Remaining production mainly from United States, Morocco, and Western Sahara
- Morocco has about 75% world’s total phosphate rock reserves
- Remaining production mainly from United States, Morocco, and Western Sahara
- Largest U.S. deposits in Florida, Idaho, North Carolina, and Utah
- Florida deposits found in 1880’s
- Produces about 75% of U.S. phosphate rock
- Produces about 25% of total world phosphate rock
- Florida deposits found in 1880’s
- China, United States, Morocco, Russia and India are leading consumers of phosphate rock
- About 85% used to manufacture phosphate fertilizers
- Remaining 15 percent used to make elemental phosphorus, animal feed supplements, or applied directly to soils.
- Rock phosphate can be applied directly as fertilizer
- Common in third-world countries.
- Used in organic crop production systems
- Product cost per ton is low
- Processing is minimal.
- Requires high application rates
- Has high shipping costs
¶ D. Rock phosphate characteristics
- Typically contains contain about 14% to 16% total phosphorus
- About 33% to 36% P2O5[1]
- Apatite compounds not water soluble
- Rock phosphate has no water soluble phosphorus
- Phosphorus content is not directly available to plants
- Rock phosphate must be applied to acidic soils to become effective
- Acidic soils dissolve the phosphate in this general chemical reaction:
- Ca10(PO4)6F2 + 12H+ ⇔ 10 Ca2+ + 6H2PO4- + 2F-
- Becomes much less effective in soils above pH 5.5
- Often applied directly in tropical countries
- Ineffective in neutral to alkaline soils.
- Could require months, even years to become effective agronomic phosphorus source
- Modern processing yields phosphate fertilizer materials with 90% to 100% water solubility for immediate utilization by growing crops.
¶ E. Processing phosphate rock
- Rock deposits usually covered by thick layer of overburden
- Surface mining begins by stripping off and stockpiling, overburden.
- Draglines, bucket wheel excavators, power shovels, earthmovers used to remove overburden.
- Stockpiled overburden later used to reclaim mined land
- Rock is ground to fine particles, followed by “beneficiation” process
- Beneficiation can require several steps.
- Process removes impurities
- Impurities include sand, clay, carbonates, organics, and iron oxide.
- Concentrates phosphate resulting in phosphate rock product with about 23% to 37% P2O5
- “Calcination” process removes organic matter at some facilities
- Passes rock ore through furnace to remove organic matter
¶ F. What is phosphoric acid (H3PO42-)?
- Produced from beneficiated phosphate rock
- Base product for most commercial phosphate materials
- Produced by two processes
- Wet process
- Thermal process
- Can be applied directly as fertilizer material by itself
- Is corrosive
- Is difficult to store, handle, and apply
- Often processed further to produce other phosphate fertilizer materials
¶ G. Wet Process Phosphoric Acid
- Produced by treating the phosphate rock with acid
- Usually treated with sulfuric acid (H2SO4)
- H2SO4 produced using molten sulfur
- Resulting product often called “green acid” or “black acid”
- Nitric acid (HNO3) sometimes used
- Not commonly used in U.S.
- Usually treated with sulfuric acid (H2SO4)
- Reaction during sulfuric acid treatment produces byproducts
- Gypsum (calcium sulfate)
- Removed by filtering
- Hydrofluoric acid
- Gypsum (calcium sulfate)
- Product may contain trace impurities from original rock and from sulfuric acid.
- Impurities include:
- Carbon (from charred organic matter)
- Iron, aluminum, calcium, and fluoride
- Phosphoric acid may have color ranging from black, gray, amber, or green
- Example: black phosphoric acid has about 0.1% to 0.2% carbon as impurity
- May also color fertilizer products made from this acid
- Impurities do not affect the agronomic nutrient value of the subsequent fertilizer products.
- Liquids with fewer impurities may have fewer storage problems
- Can be purified to yield phosphoric acid of food-grade quality
- Impurities include:
¶ H. Three Forms of Phosphoric Acid
- Initial, unfiltered phosphoric acid
- Typically 30% to 32% P2O5
- Merchant grade phosphoric acid
- Concentrated from initial acid
- Typically 54% to 56% P2O5.
- Contains individual phosphorus molecules in “orthophosphate” form (H3PO4)
- Superphosphoric acid
- Produced by heating and evaporating merchant grade acid.
- Typically about 65% to 72%% P2O5
- Less corrosive than normal phosphoric acid
- Phosphorus present in superphosphoric acid as both orthophosphate and polyphosphate molecules[2]
- About 65% to 80% of molecules in orthophosphate form
- About 20% to 35% of molecules in polyphosphate form.
- Upon contact with soils, polyphosphates revert back to orthophosphates
¶ I. Thermal Process Phosphoric Acid
- Often called “furnace acid” or “white acid”
- Produced by burning (or smelting) phosphate rock with coke and silica in an electric arc furnace or a blast furnace
- Vapors captured during smelting are condensed in water
- Produces phosphoric acid with 54% to 56% P2O5
- Same phosphorus content as wet process acid
- Has few impurities
- Used for food-grade phosphates, detergents, and premium-grade fertilizers
- Produces phosphoric acid with 54% to 56% P2O5
- Produces white or clear fertilizer products
- More expensive than wet process acid
- Due to high energy cost of smelting
- Nutritionally equal to fertilizers produced by wet process
- Unbiased field research consistently shows no significant differences in agronomic performance when the two types of products are applied at same rate and same manner
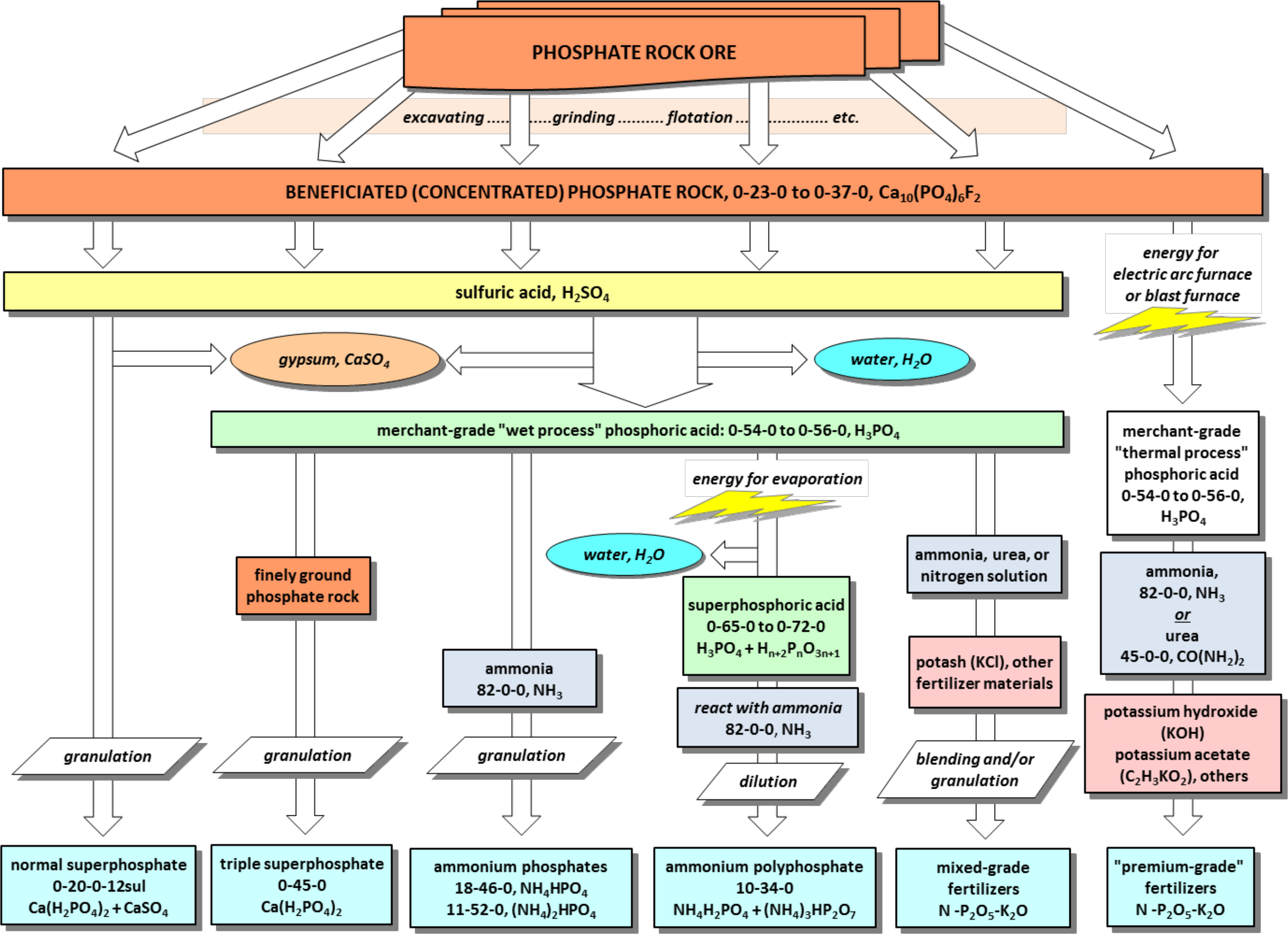
¶ J. Manufacturing Phosphate Fertilizers (see Figure 1)
- Phosphoric acid or superphosphoric acid can be combined or reacted with other materials to produce other fertilizers
- Includes both wet process and thermal process acids
- Wet process superphosphoric acid often reacted with anhydrous ammonia
- Produces ammonium polyphosphate (10-34-0, 11-37-0)
- Other materials used to manufacture other fertilizer materials include:
- Additional phosphate rock
- Urea
- Muriate of potash (KCl, 0-0-62)
- Potassium hydroxide (KOH)
- Potassium acetate (C2H3KO2)
- Others
¶ Figure 1. Phosphate Fertilizer Production Flow Chart
[1] %P2O5 = %P x 2.28 or %P = %P2O5 x 0.44
[2] Polyphosphate: series of individual orthophosphate molecules that have been chemically joined together to form “chain” of molecules
¶ References
IPNI. 2010. Phosphorus Fertilizer Production and Technology (*.ppt). October 2010 Ref. #10120 www.ipni.net accessed 01Feb2014
FIPR. 2010. Phosphate Primer. Florida and Industrial and Phosphate Research Institute, Bartow, FL. accessed 06Feb2014. www1.fipr.state.fl.us/PhosphatePrimer
The Fertilizer Institute. 2013. Fertilizer 101 Resources. 06Feb2014. http://www.fertilizer101.org/dictionary/ accessed accessed 06Feb2014
Univ. of York. 2014. Phosphoric acid. accessed 06Feb2014 .www.essentialchemicalindustry.org/chemicals/phosphoric-acid.html
Jasinski, S.M. “Mineral Resource of the Month: Phosphate Rock”. American Geosciences Institute accessed 21Mar2018 https://www.earthmagazine.org/article/mineral-resource-month-phosphate-rock
Russel, D.A. and G.G. Williams. 1977. History of Chemical Fertilizer Development. Soil Science Soc. of America Journal. 41:260-265.file:///C:/Users/fvocasek/Downloads/sssaj-41-2-SS0410020260.pdf accessed 23Mar2018