⇦ Back to Fertilizer Lime Amendment Technology and Use Home
¶ Introduction
All ammonium and ammonia‐based fertilizers have the potential to lose nitrogen through volatilization. Urea‐based fertilizers, both dry materials and liquid materials, have the greatest loss potential[1].
Volatilization depends on two things: (1) the rate which urea converts to ammonium by hydrolysis and (2) the rate at which ammonium is then converted to ammonia gas that volatilizes. This occurs with both dry urea in prill form (45‐0‐0) and urea as liquid nitrogen (e.g., 28‐0‐0 UAN solution) when applied to the soil surface. Droplets of liquid fertilizers that remain exposed on the soil surface dry out and crystallize to solid fertilizer quickly after application, so liquid fertilizers are not immune to volatilization loss.
Large losses are rare, but 20% to 30% loss of applied nitrogen can be common under optimal loss conditions. Most ammonia volatilization from urea typically occurs in the two‐week to three‐week period after application. There are several factors affecting potential for ammonia volatilization loss, including: soil moisture, precipitation, soil pH, temperature, surface residue, and soil properties.
¶ Soil Moisture and Precipitation
The most critical factors affecting volatilization are: (1) the surface moisture conditions at application and (2) the intensity and distribution of the precipitation events that follow application.
Volatilization decreases dramatically as urea is moved below the soil surface and put in contact with the top one to two inches of soil, either by soil incorporation or by movement with rainfall or irrigation.
Urea prills generally cannot get enough moisture from the air during dry conditions to completely dissolve and be moved below the soil surface. Volatilization can start in localized areas right around the urea prill or fertilizer droplet if there is enough humidity or sufficient dew to begin dissolving a portion of the urea.
The largest losses generally occur after urea is applied to a moist or snow‐covered soil surface without incorporation, then followed by a period of slow drying with little to no precipitation or irrigation. These conditions help maintain a zone of high humidity right at the very soil surface and around the urea‐based fertilizer.
A minimum of about one‐half inch (0.50") of rain or irrigation is necessary to dissolve surface‐applied urea and move it deeply enough into the soil to prevent volatilization and nitrogen loss. This amount must be applied in a single rainfall or irrigation application, not a series of smaller cumulative events, like light scattered rainfall (“splash‐and‐dash” rain shower).
For example, substantial volatilization losses can occur following a series of five 0.10‐inch showers over a couple of days when compared to a single 0.50‐inch rain falling in an hour or two. The light water applications can result in short, intense bursts of ammonia volatilization, especially if the soil surface is moist. Losses from these small bursts can accumulate, resulting in large overall losses.
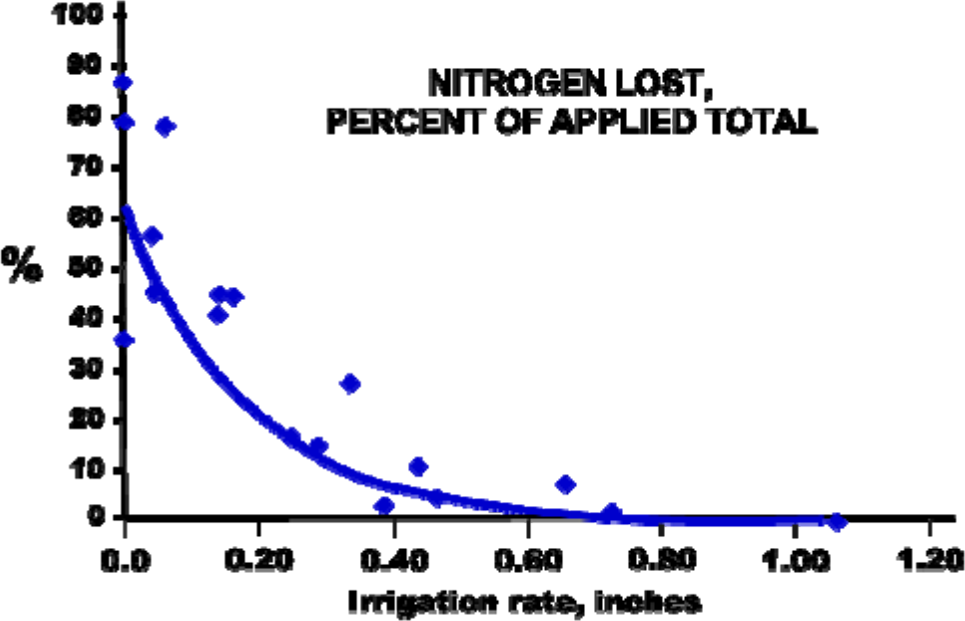
Figure 1 shows an Oregon trial where the nitrogen losses were more than 60% of the total nitrogen applied when urea was applied to a freshly irrigated soil with no subsequent irrigation and only light scattered rain over the next 30 days. An irrigation rate of 0.45 inches applied immediately after urea application reduced the loss to less than 5% of the applied nitrogen.
¶ Soil pH
Ammonia volatilization rates increase with high soil pH because the relative amount of ammonia is higher compared with the ammonium dissolved in the soil water. Ammonia is volatile and can be lost from a water solution, but ammonium is not volatile and remains in solution.
The dissolved ammonia concentration is nearly zero when the soil water pH is less than 7.5. Many soils have a surface pH lower than 7.5. However fertilizers (particularly those that are urea‐based) can elevate the soil pH right in the small zone right around the prill or droplet as they dissolve in the soil water. They may raise the soil pH enough to increase volatilization. Though the increase in pH is temporary, this can result in substantial ammonia volatilization for soils with an initial pH as low as 5.5.
¶ Figure 1. Nitrogen Loss by Ammonia Volatilization as Affected by Irrigation Application Rate
¶ Temperature
Warm soil water cannot hold as much ammonia gas as cool soil water, but urea dissolves more quickly in warmer water. The hydrolysis reaction becomes more rapid as temperature increases. For example, increasing the temperature from 45° to 60°F can double volatilization loss.
Cold soil temperatures do not ensure a halt to volatilization loss. Lower temperatures do decrease ammonia formation, but they also decrease the rate that ammonium and ammonia are converted to nitrate, the rate at which ammonium is bound to clays, or the activity of microorganisms that immobilize or tie up nitrogen. Soils also stay moist longer at low temperatures. Cooler temperatures leave ammonia available to volatilize slowly but over a longer period of time.
Field trials in Montana averaged 19% nitrogen loss through ammonia volatilization when urea was broadcast between October and April. It took at least four weeks to reach 90% of the total loss. In one trial, volatilization was still occurring 10 weeks after application.
Peak loss occurred in two‐thirds of the trials when soil surface temperatures were below 41°F. Frozen soils may actually be even at higher risk for volatilization loss. They have less ability to bind or immobilize ammonium. Also, the urea cannot move downward until the soil thaws enough.
¶ Crop Residue and Thatch
Crop residue and perennial thatch increase potential volatilization loss. This occurs because:
- (1) the urease enzyme necessary to convert urea to ammonium is produced by microorganisms that are much more active in organic material than in mineral soil,
- (2) crop resides often have a higher pH than soil,
- (3) thatch and residue may increase soil surface moisture, and
- (4) ammonia and ammonium can diffuse into and be retained by residue.
No‐till and perennial grass systems require irrigation or rainfall sooner after urea application than do bare soils or conventional tillage to minimize volatilization loss. For example, a half‐inch of rainfall within three hours of urea application on pasture can be enough to protect urea from volatilization, but a half‐inch of rain falling two days later may not.
Scattered rainfall (less than 0.10 inches) every few days on thatch or straw residue can actually cause small bursts of volatilization by increasing urea conversion to dissolved ammonia.
¶ Soil Properties
Soil properties that resist (or “buffer”) pH changes decrease ammonia volatilization. Converting urea to ammonium increases the pH around the prill or droplet, which then accelerates conversion of ammonium to ammonia. These properties include clay content, soil organic matter, and/or bicarbonate content.
Sandy soils and low organic matter soils are often considered poorly buffered. Soils with low bicarbonate content do not resist the change to higher pH levels, helping to increase volatilization potential. These conditions are considered high‐risk for volatilization loss.
Figure 2 compares the pH change and degree of ammonia loss from two different soils with different buffering capacity following urea application.
The pH rises quickly in the soil with low buffering capacity, peaking at about 4 to 6 days. The pH peak is followed closely by a rise in ammonia loss. Ammonia loss in the poorly buffered soil continues even though the pH returns to its original level.
¶ Figure 2. Soil Surface pH and Accumulated Ammonia-Nitrogen Loss as Affected by Soil Buffering Capacity
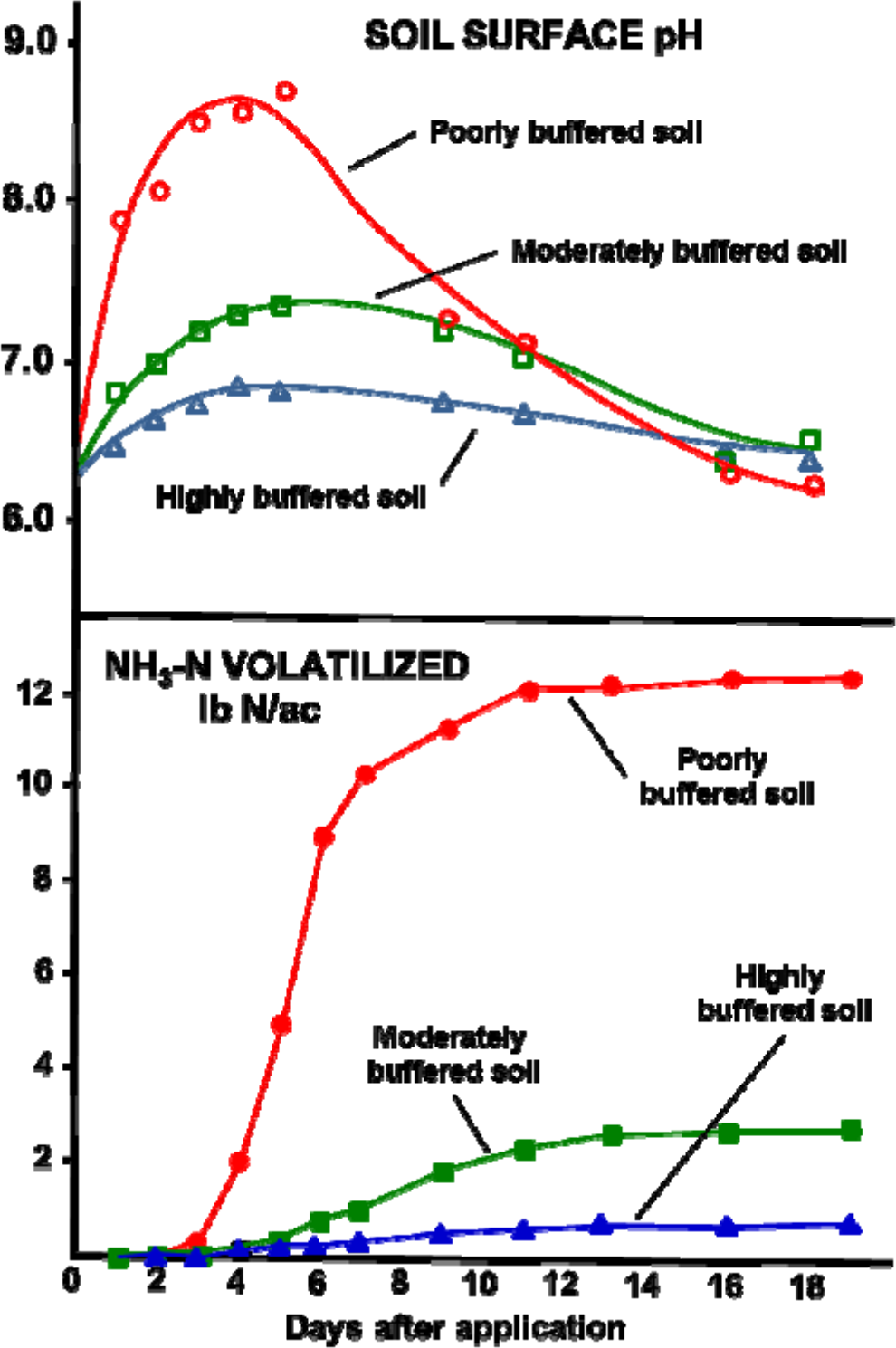
The pH changes in the moderately buffered and highly buffered soils are smaller and take longer. This corresponds directly to the comparative degree of ammonia loss for the two soils.
Soils with high cation exchange capacity reduce ammonia volatilization because ammonium (NH4+) binds to the exchange surfaces. This leaves less ammonium in solution to convert to ammonia that would then be susceptible to volatilization loss.
Volatilization losses from calcareous soils (those with “excess lime”) are typically higher for urea‐based fertilizers than for ammonium‐based fertilizers, depending on soil conditions and ammonium‐fertilizer formulation.
Because soil characteristics (like texture, CEC, and organic matter) are relatively constant over time, producers with high‐risk soil properties should pay more attention to the variables that they can control (like timing, irrigation, etc.) to minimize volatilization loss.
¶ Urea‐Based Fertilizer Management
Producers should evaluate the risk for volatilization loss by considering the field conditions at application. Table 1 lists factors that contribute to high‐risk and low‐risk conditions. The practices that minimize or prevent volatilization include:
- (1) Delaying application under high risk‐conditions when feasible
- (2) Incorporating urea‐based fertilizers within one to two days after application by
- a tillage operation of deeper than two inches, an irrigation of one‐half inch or more, or
- applying before an expected rainfall of more than one‐half inch in a single event.
- (3) Banding fertilizer deeper than 2 inches
- (4) Adding NBPT to surface‐applied urea
- (5) Using a controlled‐release nitrogen fertilizer
¶ Fertilizer Source
Ammonia volatilization from different fertilizers varies because of the form of nitrogen they contain, how they are applied, and how they interact with soils. Urea‐based fertilizers, anhydrous ammonia, and aqua ammonia have high volatilization potential because they can increase soil pH sufficiently in the application zone to increase ammonium conversion to dissolved ammonia.
Ammonium sulfate has higher rates of volatilization in calcareous soils (those containing free lime), than acidic soils because the sulfate ion dissolves some calcium carbonate, increasing pH in the application zone. Ammonium nitrate has low volatility because it has little effect on soil pH.
Urea granules that are dispersed rather than applied in concentrated bands have less localized effect on pH and lower volatilization loss.
Sprayed liquid urea may have lower volatilization potential than granular urea because it leaves a thin, even distribution of urea, rather than areas with high urea concentration.
Surface applied urea‐ammonium‐nitrate solution (UAN 28‐0‐0 or 32‐0‐0) has generally demonstrated lower volatilization loss than granular urea. This is probably because it is diluted by surface broadcasting. Also, one‐fourth of the nitrogen in the applied UAN solution is in the nitrate form, which has no chance of volatilizing.
¶ Enhanced Urea Fertilizers
Manufacturers have developed products to reduce nitrogen losses and increase nitrogen use efficiency. The impact of these specialized fertilizer products on crop yields varies with the soil and environmental conditions. These products may have lower volatilization loss than conventional urea, but may not produce a proportionate yield increase. Nitrogen availability is one of many factors affecting final yields.
These fertilizer enhancement products do not completely stop volatilization losses. They can delay the process long enough for mechanical incorporation, irrigation, or sufficient rainfall to move surface‐applied urea below the soil surface, which minimizes volatilization loss.
Urea fertilizer can be stabilized with N‐(n‐butyl) thiophosphoric triamide (NBPT), the active ingredient in Agrotain®. NBPT prevents hydrolysis of urea to ammonium by inhibiting urease, an enzyme necessary for the process. It does not prevent, but can delay volatilization, reducing ammonia losses under many conditions.
Controlled‐release products, such as polymer‐coated urea (e.g., ESN®) have volatilization losses similar to well incorporated granular urea. The coating keeps the urea from direct contact with the soil, but gradually breaks down and slowly releases urea into the surrounding environment. This can delay ammonia formation until after the urea has been soil incorporated.
Maleic‐itaconic co‐polymer salts (e.g., Nutrisphere®) are claimed to inhibit urease, thus delaying volatilization loss. Laboratory experiments clearly show that no urea inhibition occurs when these products are used at label rates.
¶ Placement and Timing
Avoid broadcasting urea without incorporation whenever possible. When not possible, consider delaying application until a half‐inch irrigation or rain event is likely. Timing urea application so that it can be followed shortly by incorporation may be more important than applying urea on dry rather than moist soils.
Adequate tillage is needed for incorporating urea effectively to about two inches below the soil surface. For example, seeding with air drills after broadcasting does not incorporate granular urea enough to reduce volatilization.
Subsurface banding can help reduce volatilization from urea, but bands should be at least two inches below the surface to be effective. Closing the application slot made by the banding or knifing can trap ammonia produced by the concentrated urea band. Volatilization losses may be higher if urea is banded in wet clay or in dry, coarse soil because the concentrated urea band and the atmosphere may be in direct connection.
Urea may be converted to ammonia faster in the subsurface due to generally moister conditions than on the surface.
¶ Summary
Soil moisture at application and the rainfall or irrigation after application can play the greatest roles in affecting volatilization. Cold temperatures alone do not protect broadcast urea from volatilization loss.
Evaluate soil and field conditions to identify the risk level for volatilization losses. The risk of nitrogen loss by volatilization increases as the number of high‐risk conditions increases. Soil moisture is likely to be the most important risk condition.
After identifying the risk level, use the best management practices to minimize potential losses. These practices include:
- Incorporating urea with equipment, irrigation or rainfall (one‐half inch or more in a single event);
- Adding NBPT to surface‐applied urea or using a controlled release fertilizer; and
- Avoiding broadcast applications under high‐risk conditions unless there is opportunity to incorporate the urea within one to two days of application
[1] ammonium = NH4+; ammonia = NH3; urea = CO(NH2)2
¶ References
Ferguson, R.B., D.E. Kissel, J.K. Koelliker and Wes Basel. 1984. Ammonia volatilization from surface‐applied urea: Effect of hydrogen ion buffering capacity. Soil Sci. Soc. Am. J. 48:578‐582. for Field Crops.
Jones, C., R. Engel., D. Horneck, and K. Olson‐Rutz. 2012. Minimizing urea volatilization in cool semi‐arid regions. Crops & Soils. 45:28‐32
Kissel, D.E. 2005. Ammonia Volatilization from Urea: How large is the issue and losses? PPT file, Univ. of Georgia.
Franzen, D.W. Nitrogen Extenders and Additives for Field Crops. Pub. SF‐1581. Coop. Ext. Serv., North Dakota State Univ., Fargo ND. 8 pg
Bock, B.R. and D.E. Kissel. 1988. Ammonia volatilization from urea fertilizers. National Fertilizer Development Center, Muscle Shoals, Alabama. 189 pg.