⇦ Back to Soil Fertility and Plant Nutrition Home
¶ A. Molybdenum in the Plant
- Considered an essential micronutrient
- Required in smaller amounts than any other essential nutrient
- Typical plant concentrations between 0.5 and 2.0 ppm Mo
- Common foliar sufficiency range is between 0.15 and 0.30 ppm Mo
- Uptake by plant roots as MoO42- (molybdate) anion
- Translocated preferentially to leaves
- Moderately mobile in plant
- Essential component of two critical enzymes
- Nitrate reductase
- Nitrate provides most of the plant nitrogen requirement
- Amino nitrogen is form found in proteins
- Nitrate reductase enzyme required for reduction of nitrate (NO3-) to amino-nitrogen (-NH2) for protein synthesis
- Nitrogenase
- Enzyme required by microorganisms for fixation of atmospheric nitrogen (gas N2) to amino-nitrogen (-NH2)
- Needed by Rhizobium, Azotobacter, blue-green algae, others
- Nitrate reductase
- Also involved in iron uptake and translocation
- Deficiency symptoms
- Symptoms usually appear in whole plant
- Various symptoms, depending on plant species
- Yellowing, stunting, interveinal mottling
- Cupping of older leaves, necrotic spots on leaf tips and margins
- Whiptail
- Limited expansion, deformation of young leaf blades
- Brassica species; i.e., cauliflower and other crucifers
- Nitrogen deficiency symptoms occur in legumes when molybdenum is inadequate for N-fixation
¶ Figure 1. Molybdenum Deficiencies
¶ B. Molybdenum in the Soil
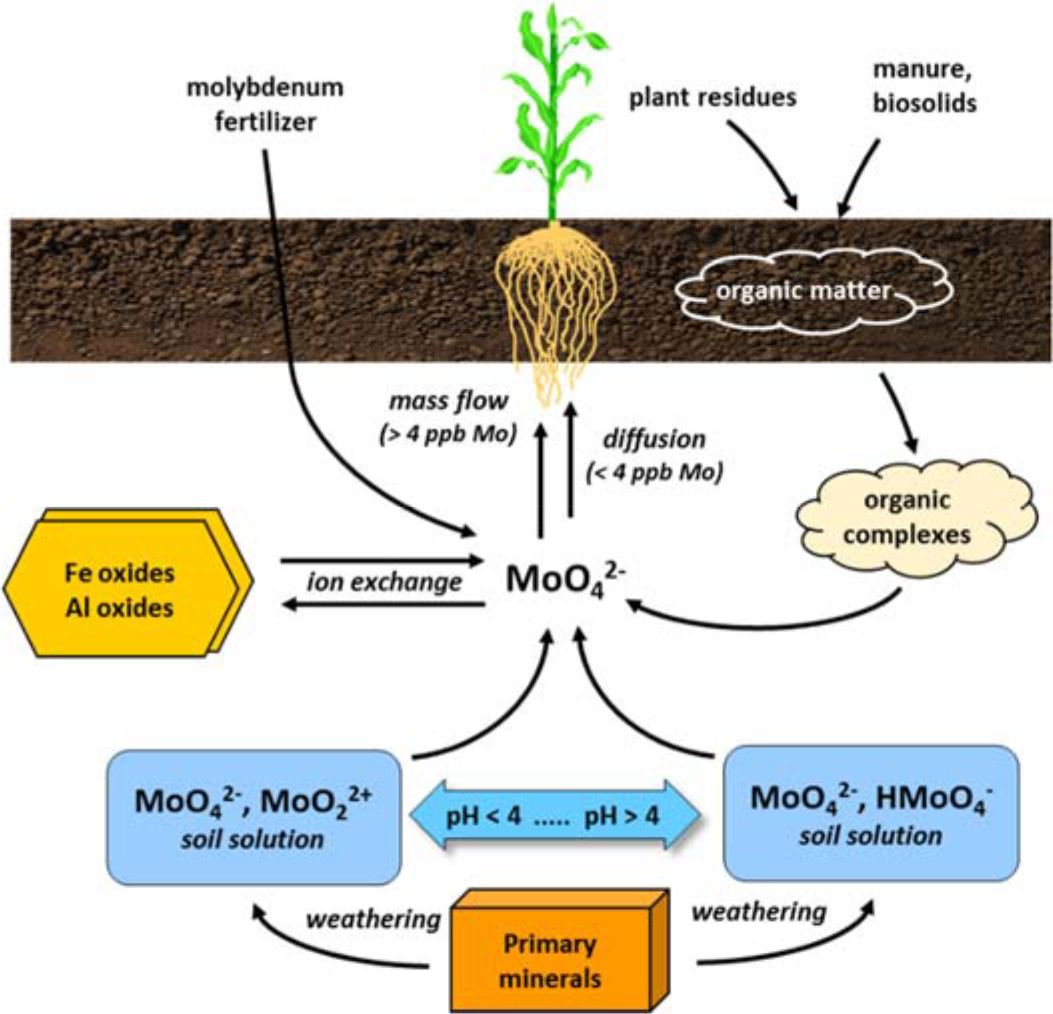
- Molybdenum moves to roots by mass flow and diffusion
- Generally moves by mass flow
- Diffusion important in low molybdenum soils (less than 4 ppb Mo in solution)
- Average total content in soil is about 2 ppm Mo
- Ranges between 0.2 and 5 ppm Mo
- Molybdenum sources
- Soil solution molybdenum
- Form is affected by solution pH
- Primary and secondary minerals containing non-exchangeable molybdate
- Soils derived from shale and granite tend to have higher molybdenum content
- Soils derived from sands tend to have low molybdenum content
- Exchangeable molybdenum held on surfaces of iron and aluminum oxides
- Organically bound molybdenum
- Small amounts of molybdenum in organic matter
- Higher organic matter soils generally have higher molybdenum availability
- Soil solution molybdenum
- Strongly adsorbed to iron-oxide and aluminum-oxide surfaces
- Similar to phosphate adsorption
- Portion of molybdenum becomes unavailable to plant
- Soils with high iron tend to be low in available molybdenum
- Less tightly adsorbed than phosphate
- Can be displaced from adsorption surfaces by high phosphate in soil solution
- Not adsorbed by calcium compounds in alkaline, calcareous soils
- Similar to phosphate adsorption
- Soil solution molybdenum
- Very low concentrations in solution
- Found predominantly as:
- Molybdate, MoO42-
- Most common form
- Hydrogen molybdate, HMoO4-
- iii. Molybdic acid, H2MoO40
- Molybdate, MoO42-
- Soil pH is important factor in availability
- Molybdenum becomes more available as soil pH increases
- Solubility increases 10 times with each 1 unit increase in soil pH
- Opposite of most micronutrients
- Molybdate acts as a weak acid
- MoO42- becomes more dominant as pH increases
- pH < 4: H2MoO40, MoO42-
- pH > 4: HMoO4-, MoO42-
- Molybdenum becomes more available as soil pH increases
¶ Figure 2. Molybdenum Sources
¶ C. Molybdenum Deficiency Conditions
- Deficiencies occur in acid soils and soils with high iron-oxide, aluminum-oxide contents
- Most frequently found in acid sandy soils of Atlantic Coast and Gulf Coast
- Deficiencies rare in other areas
- Nutrient interactions
- Uptake inhibited by high sulfate
- Possible ion antagonism, SO42- vs. MoO42-
- Sulfate ion may compete with molybdate ion for root surface adsorption sites
- pH effects of nitrate sources
- Nitrate increases, ammonium decreases molybdenum uptake
- Conversion of ammonium (NH4+) to nitrate (NO3-) can lower soil pH
- Uptake inhibited by high sulfate
- Deficiency more severe under dry conditions
- Mass flow movement is reduced
- Crop have different sensitivities to soil solution deficiency
- Legumes and some vegetables more likely to respond than small grains and other grasses
- Responses more likely in acid soils (< pH 5.5)
- Potentially responsive: broccoli, cauliflower, lettuce, onion, spinach, table beet
- Moderately responsive: alfalfa, bean, cabbage, clover, pea, radish, soybean, sugar beet, tomato, turnip
- Low response: asparagus, barley, carrot, celery, corn, grass, mint, oat, potato, sorghum, small grains, sudangrass
¶ D. Molybdenum Excess
- Toxicity rare in plants
- Toxicity more likely with ruminant livestock than with plants
- Livestock toxicity called “molybdenosis”
- Imbalance of molybdenum and copper in diet
- May occur when forage molybdenum content greater than 5 to 10 ppm Mo
- Low copper availability in organic soils
- High molybdenum plants typically found in wet soils with neutral to alkaline pH
- Imbalance of molybdenum and copper in diet
¶ E. Soil Testing for Molybdenum
- Soil tests are technically imprecise
- Routine analysis not generally available
- Maintaining proper soil pH helps manage deficiency
- Soil test methods
- Ammonium oxalate extraction (pH 3.3)
- AB-DTPA extraction (ammonium bicarbonate-DTPA, pH 7.6
- Plant tissue analysis may be better alternative
- Tissue concentration of 0.1 to 1.2 ppm Mo generally considered adequate
¶ F. Molybdenum Nutrient Management
- Manure, biosolids, and other organic sources
- Low molybdenum content, but generally contain adequate amounts
- Inorganic fertilizer materials
- Sodium molybdate, Na2MoO4●2H2O
- Common analysis: 38%-46% Mo
- Ammonium molybdate, (NH4)6Mo7O24●4H2O
- Common analysis: 54% Mo
- Molybdenum sulfide, MoS2
- Common analysis: 60% Mo
- Sodium molybdate, Na2MoO4●2H2O
- Fertilizer requirements are low
- Single fertilizer application often adequate for two to four years
- Seed treatments more common because of small amounts needed
- Seed treatment rates typically less than 0.10 lb/ac
- Small amount applied with seed usually yields same as larger amount applied to soil
- Can be applied to foliage for correcting deficiencies
- Limited response to foliar application
- Liming can often eliminate deficiencies
- Increasing soil pH increases molybdenum availability
¶ References
Rosen. 2008. SOIL 3416: Plant Nutrients in the Environment. Univ. of Minnesota. Lecture 13 outline accessed 1/15/2008
http://www.soils.umn.edu/academics/classes/soil3416/lecture13.htm
Soil-Plant Nutrient Cycling & Environmental Quality, spring 1998, Oklahoma State Univ., class publication.
Hagstrom. 1977. A Closer Look at Molybdenum. Fertilizer Solutions magazine. July-August 1977.
Kelling, Schulte, Walsh. 1999. Soil and Applied Molybdenum. Pub. A3555. Univ. of Wisconsin Coop. Ext. Serv., Madison, Wisconsin. 2 pg.
Mills, H. and J. B. Jones. 1996. Plant Analysis Handbook II. Micro Maro Pub., Athens, Georgia. pg. 49-51.
Tisdale, Nelson, Beaton, Havlin. 1993. Soil Fertility and Fertilizers (5th ed.). MacMillan Publishing, New York. pg. 72-73, 346-350