⇦ Back to Soil Fertility and Plant Nutrition Home
¶ A. Chlorine or Chloride?
- “Chlorine” is name of element
- Elemental chlorine does not occur naturally
- Exists as chlorine gas (Cl2)
- Manufactured from chloride salts
- Very reactive, combines quickly with other elements
- Elemental chlorine does not occur naturally
- Chlorine exists in nature as component of chloride salts
- Typically as salt of calcium, magnesium, sodium, or potassium
- Chloride is non-reactive form found in soil
¶ B. Chloride in the Plant
- One of 16 essential plant nutrients
- Common plant levels between 50 and 200 ppm Cl
- Highest in leaf blades, followed by petioles, shoots, stems, and fruits
- Functions in plant
- Plant water relations, osmotic regulation
- Osmotic solute
- Affects degree of plant cell hydration
- Leaf turgor or “crispness”
- Helps balance charge of positive ions in cation transport
- “Counter ion” to K+,Ca2+, Mg2+, NH4+
- Required for O2 evolution in photosynthesis
- Plant water relations, osmotic regulation
- Very mobile in plants
- Taken up by roots as chloride ion (Cl-)
- Remains in chloride form within plant
- Chloride ion can also be absorbed through leaves
- Excess chloride more common problem than chloride deficiency
- Root uptake increases as concentration increases in soil solution
- Deficiency symptoms
- Wilting
- Leaf chlorosis
- Often in young leaves despite mobility
- Random chlorotic spots on wheat flag leaf
- Can be confused with tan spot disease
- Does not express in all varieties
- Inhibited root growth, excessive root branching
- Bronzing and leaf necrosis
- Symptoms may be absent in corn or sorghum
- Toxicity symptoms
- Reduced water uptake due to high soil chloride concentration
- Premature yellowing, burning of leaf tips and margins
- Thickening and rolling of leaves
- Reduced fruit and tuber quality
- Severe cases
- Bleached leaves
- Necrotic interveinal areas
- Scorched leaf margins
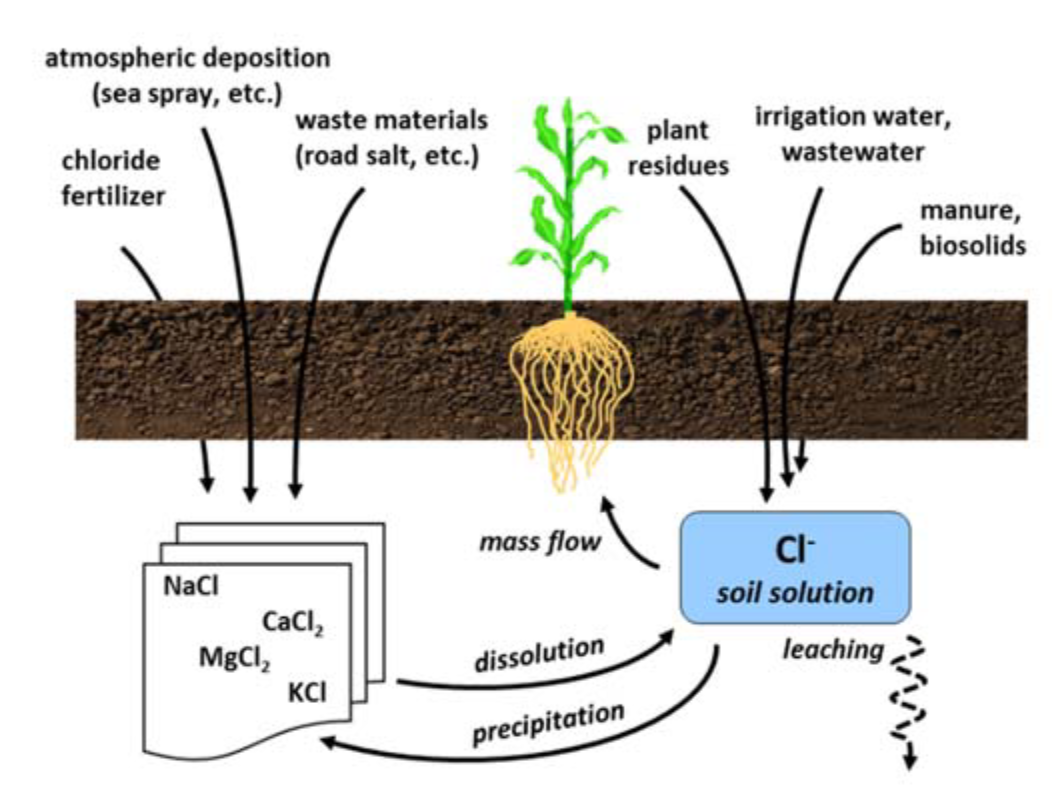
¶ C. Chloride in the Soil
- Main mineral forms are soluble chloride salts
- Earth’s crust contains about 500 ppm Cl
- Average soil concentration about 100 ppm Cl
- Very little chloride contained in organic matter
- Very mobile, readily leached
- Chloride ion (Cl-) moves to roots by mass flow
- Not adsorbed by soil colloids
- Sometimes used as tracer ion for nitrate or sulfate movement
- Some adsorption in extremely acid soils
- May compete with nitrate for exchange sites on root surfaces
- Major anion in saline soils
- Accumulations more common in arid regions
- Accumulates above shallow water tables
- Poor internal drainage keeps soils wet
- Chloride added through irrigation water, but inadequately leached to remove
- Accumulations more common in arid regions
- Added to soil by precipitation, especially in coastal regions
¶ Figure 1. Chloride Sources
¶ D. Chloride Deficiency Conditions
- Chloride in soil is readily soluble and highly available
- Moves readily with water
- Root uptake increases as soil concentration increases
- Highly leached soils sometimes deficient
- Sandy soils
- High precipitation in fall and winter months
- Soils naturally low in chloride
- Little atmospheric deposition in areas that are long distances from ocean coasts
- Nutrient interactions
- Nitrate and sulfate can inhibit chloride uptake
- Helps to suppress certain root and leaf diseases
- Exact mechanism of suppression unknown
- May be related to osmotic regulation
- May restrict nitrate uptake
- May create less favorable pH for pathogens due to increased ammonium-nitrogen uptake
- Documented in small grains and vegetable
- Winter wheat: take-all, tan spot, stripe rust
- Spring wheat: leaf rust, tan spot
- Barley: common root rot, Fusarium root rot
- Corn: stalk rot
- Potatoes: hollow heart, brown center
- Celery: Fusarium yellows
- Exact mechanism of suppression unknown
- Plant analysis critical levels
- Wheat
- Flag leaf at boot stage
- Less than 0.10% to 0.12% Cl
- Corn or sorghum
- 6-leaf to 8-leaf stage
- Youngest fully-emerged leaf
- Less than 0.10% to 0.12% Cl
- Wheat
¶ E. Chloride Toxicity Conditions
- Excess chloride more common problem than deficiency
- Problems more common in semi-arid areas
- Limited precipitation not able to remove chloride accumulations in soil
- Naturally occurring accumulations
- Saline soils develop in “seep” areas, “pan spots”
- Ground water surface close to soil surface
- Accumulations can develop in more humid areas with favorable soil conditions
- Manmade accumulations
- High chlorides in irrigation water
- Deicing salt (sodium chloride)
- Waste and wastewater disposal
- High chloride fertilizer rates (e.g., potash)
- Petroleum, natural gas exploration wastes
- Problems more common in semi-arid areas
- Chloride can depress plant growth in two ways
- “Salt effect”
- Excessive total ion accumulations, including chloride
- High concentration increases soil osmotic pressure
- Reduces water availability to roots
- Direct toxicity
- Can be absorbed by roots, translocated to leaves
- Entry through leaf cuticle
- Sprinkler irrigation deposits droplets of high chloride water on leaf surface
- Damage more pronounced under hot, dry, windy conditions
- “Salt effect”
- Large differences in plant sensitivity
- Some crops are specifically sensitive to the chloride ion
- Toxicity common with foliar levels of 0.5% to 2.0% Cl
- Halophytes (“salt loving” plants) may contain up to 4% Cl in foliage
- Sensitive: berries, fruit trees, beans, cotton, tobacco
- See Crop File 4.03.014 Toxic Ions in Salt-Affected Soils
- Some crops are specifically sensitive to the chloride ion
¶ F. Soil Testing for Chloride
- Precision of analysis is limited
- Expect +/- 15% to 25% variation between individual analyses
- Duplicate or triplicate analysis recommended to verify results
- Salinity evaluation
- Extracted with water using saturated paste method
- Used as diagnostic tool to identify toxicity potential
- Analysis for nutrient requirement
- Mercury (II) thiocyanate method
- Most common method
- Extracted with calcium nitrate solution
- Determined colorimetrically
- Ion exchange chromatographic method
- Extracted with calcium hydroxide
- Mercury (II) thiocyanate method
- Surface and subsoil samples to 24 inch depth recommended for nutrient management
- Critical levels: wheat, corn, sorghum
- Soil analysis: Less than 30 to 45 lb Cl/ac (4 to 6 ppm Cl) in 24-inch depth
¶ G. Chloride Nutrient Management
- Organic, biological sources
- Chloride readily leached out of crop residue
- Chloride levels usually low in dry manure and other dry organic sources
- Significant amounts can be removed by leaching as material is weathered during storage and handling
- Typical content about 5 to 10 lb Cl per ton
- Chloride concentrations may be comparatively high in liquid manures or wastewater
- Chloride salts included in livestock diets; included as waste from municipal or commercial processes
- Liquid storage structures retain added chlorides
- Chlorides may become concentrated as water evaporates from structure
- Potential for “salt” injury if material is soil applied at high rates or in proximity to seedling plants
- Potential for foliar injury if applied through sprinkler
- Inorganic sources
- Potassium chloride solid, KCl
- Muriate of potash, 0-0-60 or 0-0-62
- Common analysis: 45% to 47% Cl
- Magnesium chloride, MgCl2
- e.g., Chlori-Mag®
- Common analysis: 7% Mg, 22% Cl
- Ammonium chloride, NH4Cl
- Common analysis: 25% N, 66% Cl
- Calcium chloride, CaCl2
- Common analysis: 34 % Ca, 58% Cl
- Irrigation water
- Each 1 mg/L Cl equivalent to 0.2 lb Cl per acre-inch
- Potassium chloride solid, KCl
- Fertilizer application
- Agronomic crops
- Rates: 10 to 30 lb Cl/ac
- Wheat: apply before planting or make topdress application after emergence
- Corn, sorghum: apply preplant or at planting
- Soil incorporation not required
- Broadcast or banded applications equally effective
- Caution if placing chloride-containing fertilizers in-furrow with seed or close to seed
- Can be compatibility problems if mixing chloride fertilizer solutions with UAN or phosphate liquids
- Calcium chloride solution used as preharvest treatment to reduce physiological disorders
- Bitter pit in apples
- Blossom-end rot in tomatoes
- Agronomic crops
¶ References
Rosen. 2008. SOIL 3416: Plant Nutrients in the Environment. Univ. of Minnesota. Lecture outline accessed 1/15/2008
http://www.soils.umn.edu/academics/classes/soil3416/lecture13.htm
Mills, Jones. 1996. Plant Analysis Handbook II. Micro Macro Pub., Athens, Georgia. pg. 39-41.
Soil-Plant Nutrient Cycling & Environmental Quality, spring 1998, Oklahoma State Univ., class publication.
Tisdale, Nelson, Beaton, Havlin. 1993. Soil Fertility and Fertilizers (5th ed.). MacMillan Publishing, New York. pg. 73-75, 342-346
Schulte. 2004. Soil and Applied Chlorine. Pub. A3556. Univ. of Wisconsin Coop. Ext. Serv., Madison, Wisconsin. 2 pg.
Lamond, Leikam. 2002. Chloride in Kansas: Plant, Soil, and Fertilizer Considerations. Pub. MF-2570. Kansas State Univ. Coop., Ext., Manhattan KS.