⇦ Back to Soil Fertility and Plant Nutrition Home
¶ A. Boron in the Plant
- Considered an essential micronutrient; required by plants in very small amounts
- Typical crop removals range from 0.05 to 0.45 lb B/ac
- Unevenly distributed within plant
- Highest levels in reproductive parts (anthers, stigma, ovaries)
- Leaf content varies with plant type
- Monocot species range between 1 to 6 ppm B
- Dicot species ranges between 20 to 70 ppm
- Uptake by plant roots primarily as H3BO3 (boric acid)
- Uptake is passive, primarily by mass flow
- Required for new vegetative growth and reproductive development
- Sugar transport
- Membrane permeability; component of cell walls
- Pollen germination and pollen tube growth
- Cell elongation, division, and differentiation
- Much of the boron requirement is extracellular, in free spaces of cell walls
- Required for apoplastic (“structural”) functions; e.g., cell walls, lignification, xylem differentiation
- Apoplastic nature of boron similar to calcium
- Apoplast composed of Plant’s non-living parts
- Boron also has metabolic functions.
- Required for apoplastic (“structural”) functions; e.g., cell walls, lignification, xylem differentiation
- Not readily translocated in plant
- Deficiencies occur first in growing points, young leaves
- Generally immobile in phloem
- Importance of xylem translocation (transpiration stream) for distribution in plant is similar to calcium
- Xylem transport to leaves is “one-way ticket”
- Cannot reenter phloem from leaves of many species once delivered by xylem
- Can be transported by simple sugars (polyols) in photosynthesis
- Forms organic complex with polyol molecule
- Transported by phloem stream to actively growing regions of plant
- Cannot reenter phloem stream if plant species does not produce significant amount of polyols
- Deficiency symptoms
- Cessation of terminal bud growth and root tips
- Leaf chlorosis, death of youngest leaves
- Shortened internodes, “rosetting”
- rosette = circular arrangement of leaves, with all the leaves at a similar height
- Misshapen leaves; thickened stems and petioles
- Flower abortion, poor seed set and fruit development
- Characteristic crop deficiency symptoms
- Alfalfa: death of growing tip, rosetting (bushy appearance); yellowing of top leaves
- Apple and tomato: internal “corking”
- Cabbage: internal breakdown of head
- Cauliflower: deformed foliage and browning of the curd
- Celery: browning mottling of bud leaves and cracking of the stem
- Sugar beet: heart rot, crown rot, dark spots on root
- Excess boron
- Relatively narrow range between deficiency and toxicity
- Symptoms
- Chlorosis of leaf tips and margins
- Leaf scorch and burn (necrosis)
- Premature leaf drop
- Species sensitive to toxicity include: peach, grapes, fig
¶ Figure 1. Boron Deficiencies
¶ B. Boron in the Soil
- Tourmaline - a borosilicate primary mineral
- Total soil content ranges from 2 to 200 ppm B; common range 7 to 80 ppm B
- Available range typically 0.4 to 5.0 ppm B
- Organic matter sources
- Boron is constituent of soil organic matter
- Organically complexed boron is large potential source
- Adsorbs to clay and iron-oxide, aluminum-oxide surfaces
- Soil solution boron
- Boron has high affinity for oxygen
- Between pH 5 and 9, non-ionized form, boric acid (H3BO3) is dominant form in solution
- Other forms may be present in much smaller amounts; e.g., B4O7-2, H2BO3-, HBO3-2, or BO3-3
- Boron solubility primarily controlled by adsorption-desorption from mineral surfaces
- Solution pH and amount of clay, oxides, and organic matter are important factors in boron availability
- Boron retention higher at high pH
- At pH > 9.2, H3BO3 can hydrolize to H4BO4
- Boron moves to roots by passive processes
- Majority by mass flow in many soils
- Diffusion very important in low boron soils
¶ Figure 2. Born Sources
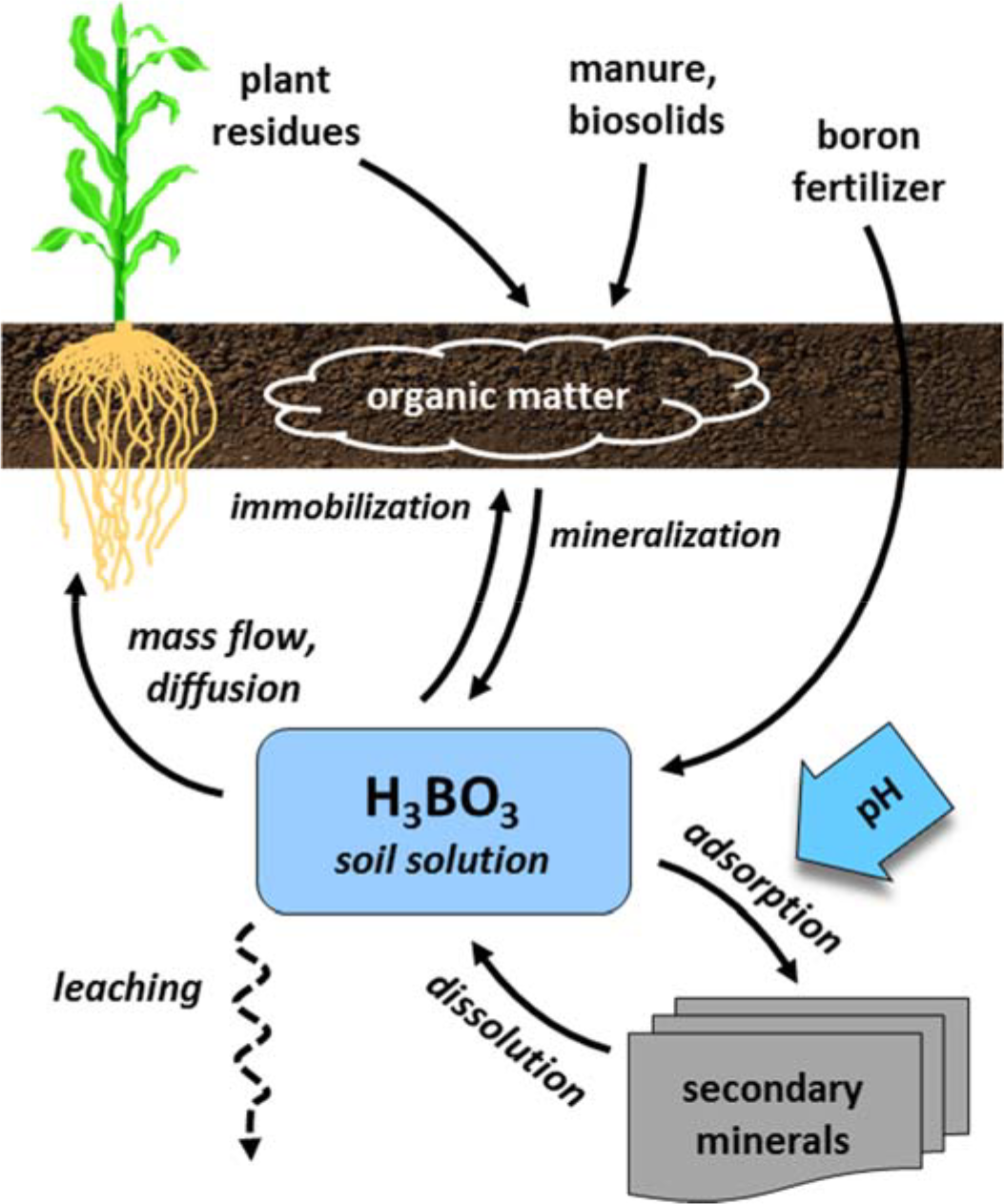
¶ C. Boron Deficiency Condition
- Boron is most available in acid soils
- Becomes strongly adsorbed to oxides and to organic matter above pH 6.5
- May leach from acid, sandy soils
- Boron is highly mobile, somewhat similar nitrate or sulfate
- Less adsorption in acidic soils, so greater chance for leaching
- Deficiencies due to low amounts of boron, even though availability is high
- Organic matter is an important reservoir of boron
- Deficiencies may occur with conditions that limit mineralization
- Irrigation water may be significant source
- Some water sources have very low boron content; e.g., snowmelt water, Nebraska Sandhills ground water
- Can reach toxic levels in certain water sources
- Dry soil conditions can limit boron availability
- Most boron passively absorbed in water uptake
- Similar to calcium
- Distributed through plant in the transpiration stream
- Continuous uptake and translocation in xylem is important
- Less water, less boron uptake
- Dry conditions decrease organic matter mineralization that releases boron
- Most boron passively absorbed in water uptake
- Nutrient interactions
- High calcium and potassium can induce deficiency
- Crops differ in boron requirement
- High requirement: alfalfa, beet, canola, cauliflower, celery, sunflower, tomato, birdsfoot trefoil, forage brassicas
- Medium requirement: apple, asparagus, broccoli, Brussels sprout, cabbage, carrot, lettuce, melons, radish, red clover, spinach, tobacco, vetch
¶ D. Boron Toxicity conditions
- More common in arid and semi-arid areas
- Soil accumulations from high boron irrigation water
- Concern when water concentrations greater than 1.0 to 2.0 mg/L B
- Composted municipal waste applications
- Borate compounds used in non-bleach laundry detergents
- Banded fertilizers placed with or close to seedlings
- Acid soils
- Low calcium increases boron sensitivity
- Liming can reduce boron uptake; reduce toxicity potential
¶ E. Soil Testing for Boron
- Hot-water soluble extractable boron (HWS-B)
- Single element extraction (only boron)
- Used to identify deficient condition
- Critical level for most crops, 0.3 to 0.5 ppm HWS-B
- Potential soil toxicity above 4.0 to 5.0 ppm HWS-B
- Interpretation may depend on soil texture
- Mehlich extractions
- Multi-element extractant
- Used mainly in acidic soils of Eastern U.S. to extract boron
- Generally well correlated with HWS-B
- DTPA-sorbitol boron
- Multi-element extractant
- DTPA used mainly to extract zinc and iron
- Sorbitol is sugar compound used to complex boron
- Saturated paste extraction
- Diagnostic test used to identify toxic condition, not deficiency condition
- Uses water as extractant
- Water added to soil sample to reach saturation
- After equilibration, water removed using vacuum,
- Extracted water is analyzed for boron
- Toxic concentrations vary according to plant species
- See Crop File 4.03.014, Toxic Ions in Salt-Affected Soils
¶ F. Boron Nutrient Management
- Organic, biological boron sources
- Manure may supply 0.01 to 0.10 lb B per ton
- Boron released from soil organic matter
- Biosolids may contain borate compounds used in non-bleach laundry detergents
- Inorganic fertilizer materials
- Borax, sodium tetraborate decahydrate, Na4B4O7●10H2O
- Common analysis: 10% to 11% B
- Solubor, Na2B4O7•5H2O + Na2B10O16•10H2O
- Common analysis: 20.5% B
- Boric acid, H3BO3
- Common analysis: 17% B
- Fertilizer borate 48, Na2B4O7•5H2O
- Common analysis: 14.3% B
- Fertilizer borate 68, Na2B4O7
- Common analysis: 20.2% B
- Irrigation water
- Each 1 mg/L B in water equivalent to 0.23 lb B per acre-inch applied as irrigation
- Borax, sodium tetraborate decahydrate, Na4B4O7●10H2O
- Soil application
- Uniform application is important to avoid toxicity
- Avoid banded applications
- High concentrations can be toxic to seeds, seedlings, and roots
- Foliar application
- Commonly used for tree fruits
- Example: to control bitter pit in apples
- Typically require multiple applications during growing season
- Boron not mobile in plant
- Commonly used for tree fruits
¶ References
Rosen. 2008. SOIL 3416: Plant Nutrients in the Environment. Univ. of Minnesota. Lecture outline accessed 1/15/2008
http://www.soils.umn.edu/academics/classes/soil3416/lecture13.htm
Soil-Plant Nutrient Cycling & Environmental Quality, spring 1998, Oklahoma State Univ., class publication.
Kelling, Schulte, Walsh. 1999. Soil and Applied Boron. Pub. A2522. Univ. of Wisconsin Coop. Ext. Serv., Madison, Wisconsin. 2 pg.
Mahler. 1981. Essential Plant Nutrients: Boron in Idaho, Pub. CIS-1085. Idaho Coop. Ext. Serv. 6 pg.
Tisdale, Nelson, Beaton, Havlin. 1993. Soil Fertility and Fertilizers (5th ed.). MacMillan Publishing, New York. pg. 66-67, 337-342
Mills, Jones J.B. 1996. Plant Analysis Handbook II. MicroMacro Pub., Athens, GA. pg. 35-39