⇦ Back to Soil Fertility and Plant Nutrition Home
¶ A. Copper in the Plant
- Considered an essential micronutrient; required by plants in very small amounts
- Crops remove less than 0.10 lb/ac per year
- Content in most plants ranges between 2 and 30 ppm
- Common sufficiency range is about 3 to 7 ppm
- Uptake by plant roots primarily as cupric ion, Cu2+
- May also be taken up in organically complexed forms
- Component of several enzyme complexes that influence carbohydrate and nitrogen metabolism
- Copper is unique in its involvement in enzymes; cannot be replaced by any other metal
- Component of many oxidase enzymes
- Necessary for:
- Photosynthesis
- Respiration
- Lignification
- Pollen formation and fertilization
- Copper not mobile within plant
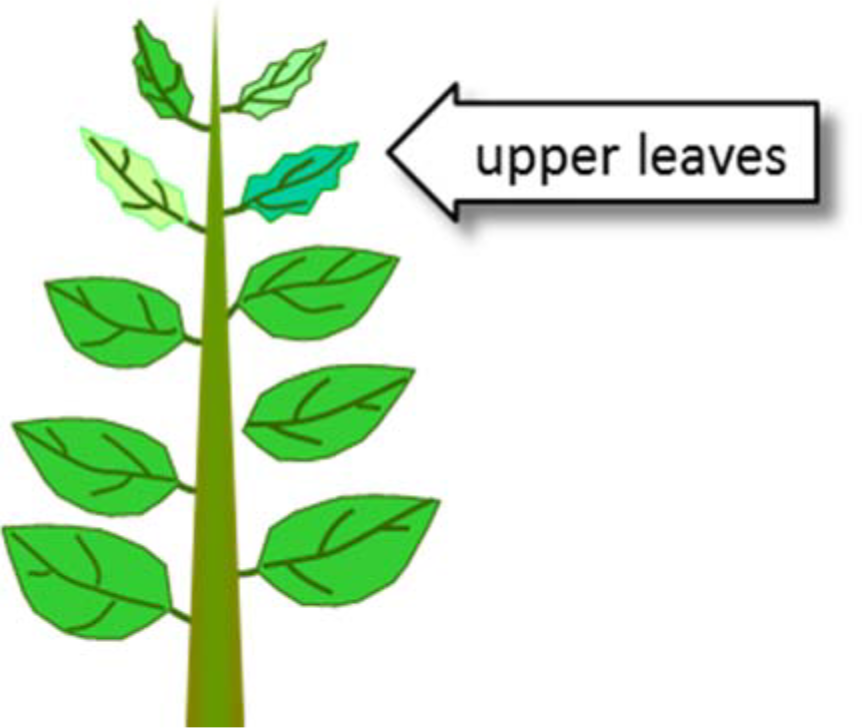
- Deficiencies occur first in growing points, young leaves
- Deficiency symptoms
- Light green, blue-green, yellowing of young leaves
- Twisted leaf tips, tip dieback
- Wilting of leaves
- Poor seed set, fruit development
- Wheat head becomes bleached, then turns gray
¶ Figure 1. Copper Deficiencies
¶ B. Copper in the Soil
- Contained in small amounts in a number of primary and secondary soil minerals
- Soil total copper content averages 30 to 50 ppm Cu
- Copper uptake
- Moves to roots by diffusion
- Uptake is active and metabolically controlled
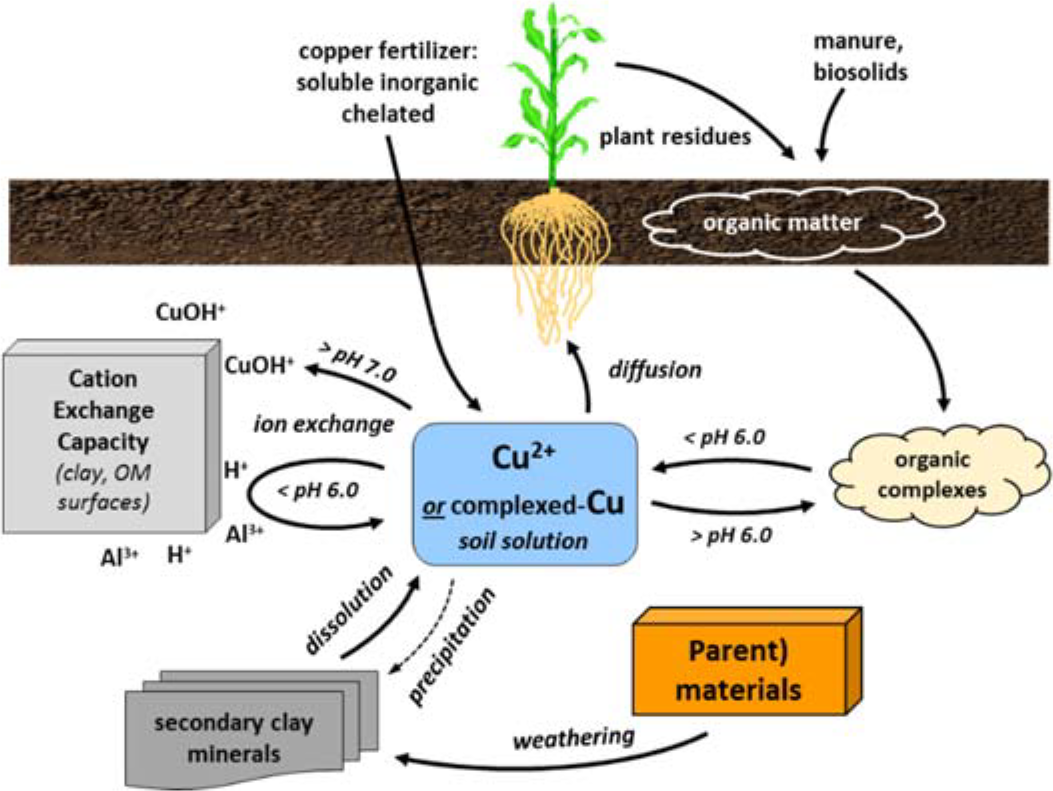
- Soil solution copper
- Immobile in soil
- Solubility primarily controlled by solution pH and adsorption on mineral and organic surfaces
- Majority of solution copper is organically complexed (chelated form)
- Copper solubility and pH
- Soil-Cu (mineral copper) + 2H+ ↔ Cu2+
- Soluble Cu2+ decreases 100 times with pH increase of 1 unit
- Copper hydrolysis reactions
- At pH > 7: CuOH+ dominant; CuOH+ adsorbs to exchange sites
- At pH 6.9 to 7.0: Cu(OH2) dominant
- At pH < 7: Cu2+ dominant
- At pH < 6: exchange sites taken up by Al3+ and H+, allowing Cu2+ to remain soluble
- Complexation with organic matter
- Availability decreases with increasing organic matter
- Soil organic matter up to 8% OM, both mineral and organic surfaces involved in adsorption
- Soil organic matter over 8% OM, most copper bound on organic surfaces
- Organic matter complexes both increase and decrease copper availability
- Chelation/complexation with low-molecular weight organic matter compounds increases solution copper
- Adsorption by insoluble, high-molecular weight organic matter decreases solution copper
- Diffusion of chelated/complexed copper important for adequate supply to plant roots
- Availability decreases with increasing organic matter
- Copper adsorption
- Adsorbed on surfaces of organic matter, clays, and oxides of aluminum, iron, and manganese
- More strongly adsorbed to oxides and more strongly bound by organic matter than any other micronutrient
- Carbonate content, soil pH, organic matter, CEC and clay content are predominant factors in adsorption
- Large amounts of copper can also be occluded or precipitated in the structure of clays and oxide minerals
¶ C. Copper Deficiency Conditions
- Deficiency most common with organic soils
- Organic soils have high adsorption capacity
- Deficiency can occur on high pH and leached, sandy soils
- Increasing pH
- Reduced copper solubility
- Increased copper adsorption
- Reduced copper availability
- Sandy soils have lower clay content and fewer cation exchange sites
- Increasing pH
- Excess iron, zinc, and phosphorus can induce deficiency
- Roots of copper-efficient crops make adaptive responses to low solution copper in manner similar to iron responses
- Biological responses include:
- Acidification
- Redox potential
- Higher rates of copper absorption and translocation
- Longer root hairs
- Biological responses include:
- Crop sensitivity to deficiency differs
- Most responsive: alfalfa, table beet, carrot, citrus, flax, lettuce, oat, onion, rice, spinach, sudangrass, wheat
- Medium response: apples, barley, sugarbeet, blueberry, broccoli, cabbage, cauliflower, celery, clover, corn cucumber, parsnip, pineapple, radish, sorghum, strawberry, sunflower, timothy, tomato, turnip
- Least responsive: asparagus, bean, canola, forage grasses, grape, pea, peppermint, pine, potato, rye, soybean spearmint, turfgrass
¶ D. Copper excess conditions
- Toxicity not common
- Depends on plant species, pH, organic matter
- May occur in limited areas with high availability
- Excess soil accumulations often due to repeated use of Bordeaux mixture (CuSO4•5H2O + Ca(OH)2) as fungicide
- Used on potatoes, snap beans, orchard crops
- Repeated applications of high-copper manures or biosolids
- Swine or poultry manure from high copper diets
- Dairy manure containing discarded copper foot-bath
- Toxicity symptoms
- Can induce typical iron deficiencies
- Stunting, reduced shot vigor
- Reduced branching, thickening
- Poorly developed, discolored roots
¶ Figure 2. Copper Sources
¶ E. Soil Testing for Copper
- Soil tests not calibrated well to plant response
- Interpretation of results may require soil pH, organic matter, soil texture, and responsiveness of crop
- Strongly recommended to verify low soil test with plant analysis
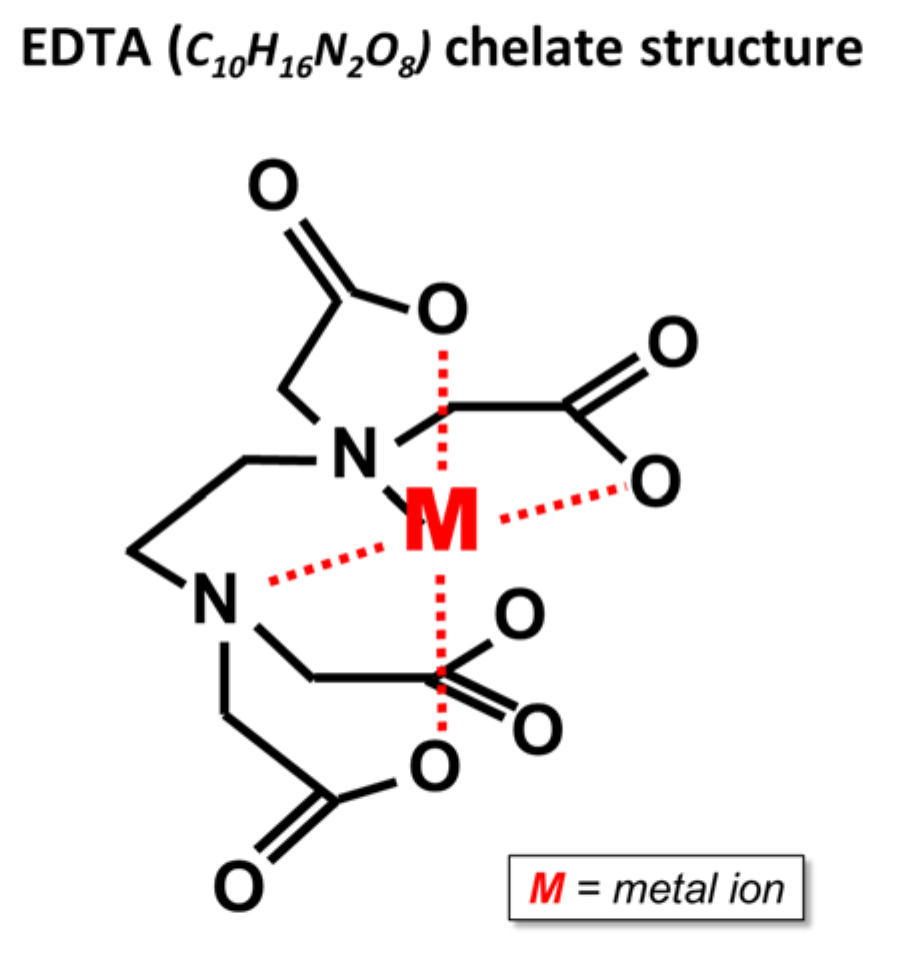
- Synthetic chelates used for copper extraction
- Act somewhat like organic chelating compounds exuded by root
- DTPA (diethylene-triamine-pentaacetic acid)
- Better adapted for neutral and high pH soils
- Used in primarily Plains and Western states
- Common critical level for mineral soils: 0.2 – 0.5 ppm DTPA-Cu
- Common critical level for organic soils: 2.5 – 4.0 ppm DTPA-Cu
- EDTA (ethylene-diamine-tetraacetic acid)
- Is component of Mehlich-3 extractant
- Used in Southeast and Eastern U.S.
- Common critical level about 0.5 ppm M3-Cu
- Acidic extractants
- Hydrochloric acid (1M HCl)
- Used for organic soils, not recommended for use on mineral soils
- Hydrochloric acid (1M HCl)
¶ F. Copper Nutrient Sources
- Organic, biological sources
- Manure usually supplies low rates of copper
- Typically ranges from 0.02 to 0.20 lb Cu/wet ton
- Some manure may supply excess copper because of copper-containing feed supplements
- Manure and biosolids also supply complex molecules which help chelate copper ions
- Manure usually supplies low rates of copper
- Inorganic fertilizer materials
- Copper sulfate, CuSO4●5H2O
- Most common source
- Common analysis: 25% Cu
- Soil and foliar applications are both effective
- Bordeaux mixture
- Mixture of copper sulfate (CuSO4) and slaked lime (Ca(OH)2)
- Used as fungicide, but contributes to soil copper concentration when washed off foliage
- Copper sulfate, CuSO4●5H2O
- Organic, non-biological materials
- Synthetic chelates
- Can be applied to soil and or as foliar treatment
- EDTA, ethylenediaminetetraacetic acid
- Common analysis: 7.5% to 13% Cu
- HEDTA, (hydroxyethyl)-ethylenediaminetriacetic acid
- Common analysis: 9% Cu
- Organic complexes
- Have chelating properties, but not as effective as synthetic chelates
- Less expensive than synthetic chelates
- Often blended with small amount of synthetic chelate to reduce cost and enhance efficiency
- Includes citric acid, lignosulfonates, polyflavinoids, etc.
- Some are industrial byproducts
- Have chelating properties, but not as effective as synthetic chelates
- Synthetic chelates
¶ G. Copper Nutrient Management
- Placement
- Surface broadcast applications must be thoroughly soil incorporated
- Can be applied in subsurface band, but may cause root injury if high rates placed too close to seed
- Foliar applications often are rescue treatment for nutrient deficiency
- May require more than one application
- Rates
- Typical broadcast rates: 2 to 6 lb Cu/ac for sensitive crops on deficient soils
- Single application may be effective for several years, depending on application rate
- Lower rates for sandy (low clay) and/or low organic matter soils (e.g., less than 6% to 8% OM)
- Typical broadcast rates: 2 to 6 lb Cu/ac for sensitive crops on deficient soils
¶ Figure 3. EDTA Chelate Structure
¶ References
Rosen, C. 2008. SOIL 3416: Plant Nutrients in the Environment. Univ. of Minnesota. Lecture 13 outline accessed 1/15/2008
http://www.soils.umn.edu/academics/classes/soil3416/lecture13.htm
Mills, H. and J. B. Jones. 1996. Plant Analysis Handbook II. Micro Maro Pub., Athens, Georgia. pg. 41-44.
Soil-Plant Nutrient Cycling & Environmental Quality, spring 1998, Oklahoma State Univ., class publication.
Tisdale, Nelson, Beaton, Havlin. 1993. Soil Fertility and Fertilizers (5th ed.). MacMillan Publishing, New York. pg. 71, 327-332
Kelling, Schulte, Walsh. 1999. Soil and Applied Copper. Pub. A2527. Univ. of Wisconsin Coop. Ext. Serv., Madison, Wisconsin. 2 pg.
IPNI. Nutri-Facts No. 10: Copper. www.ipni.net/nutrifacts accessed 04/04/2016