⇦ Back to Soil Fertility and Plant Nutrition Home
¶ A. Manganese in the Plant
- Considered an essential micronutrient; required by plants in relatively small amounts
- Typical crop removals range from about 0.1 to 0.6 lb Mn/ac
- Foliar levels range between 10 to 200 ppm Mn
- Critical foliar levels between 10 to 50 ppm Mn (dry weight basis)
- Taken up by plant roots as the manganous ion (Mn2+)
- May also be taken up in organically complexed form
- Role in photosynthesis
- Splitting of water and evolution of O2
- Manganese involved in various biochemical reactions
- Oxidation-reduction reactions, (Mn2+↔ Mn3+),
- Divalent manganous ion, Mn2+
- Trivalent manganic ion, Mn3+
- Decarboxylation
- Chemical reaction that removes carboxyl group and releases carbon dioxide (CO2)
- Hydrolysis
- Chemical breakdown of a compound due to reaction with water
- Manganese as Mn2+ can substitute for magnesium ion (Mg2+) in phosphorylation and number of other reactions
- Oxidation-reduction reactions, (Mn2+↔ Mn3+),
- Manganese not readily translocated in plant
- Deficiencies occur first in upper part of plant, growing points, and young leaves
- Manganese accumulated in leaves cannot be remobilized, while manganese in roots and stems can be remobilized
- Deficiency symptoms
- Interveinal chlorosis of young leaves
- Symptoms similar to iron deficiency
- Spotty discoloration of leaves
- Gray speck of oats, marsh spot of peas, speckled yellows of sugar beets
- Interveinal chlorosis of young leaves
- Excess manganese
- Manganese toxicity can occur on very acidic soils
- Brown/black spots (precipitated or “crystallized” manganese dioxide, MnO2) with chlorotic ring, occurring on older leaves
- Induced deficiency of iron, magnesium, calcium
¶ Figure 1. Deficiencies of Manganese
¶ B. Manganese in the Soil
- Found in primary minerals, clays, oxides, hydroxides
- Total soil concentration commonly ranges from 20 to 3000 ppm Mn, averaging about 600 ppm Mn
- Soil solution manganese
- Solubility primarily controlled by solution pH, redox conditions, and adsorption on organic surfaces
- Soluble manganous ion (Mn2+) activity decreases 100-fold for each 1 unit increase of soil pH
- Poor aeration increases manganese availability
- Soil waterlogging (saturation) reduces free oxygen (O2), lowers redox potential, which increases soluble Mn2+
- Soil compaction reduces both air exchange and water movement through soil; encourages water saturation and waterlogging
- Some Mn2+ ions desorb in exchangeable form from clay exchange surfaces
- Majority of solution manganese is in chelated form
- Manganese solubility
- Soil solution manganese controlled by solubility of MnO2
- Oxidation reduction reactions
- MnO2 + 4H+ + 2e- ↔ Mn2+ + 2H2O
- Reduction of MnO2 to Mn2+ affected by microbial activity
- Increasing pH favors oxidation of Mn2+ to Mn4+ (MnO2) in soil
- Increasing pH also increases manganese complexation on solid organic matter surfaces
- Manganese movement to roots
- Moves to roots by diffusion
- Exchangeable form can adsorb to clay surfaces
- Chelation
- Increases amount of manganese in soluble form
- Most manganese diffusing to roots is organically complexed
- Moves to roots by diffusion
¶ Figure 1. Manganese Deficiencies
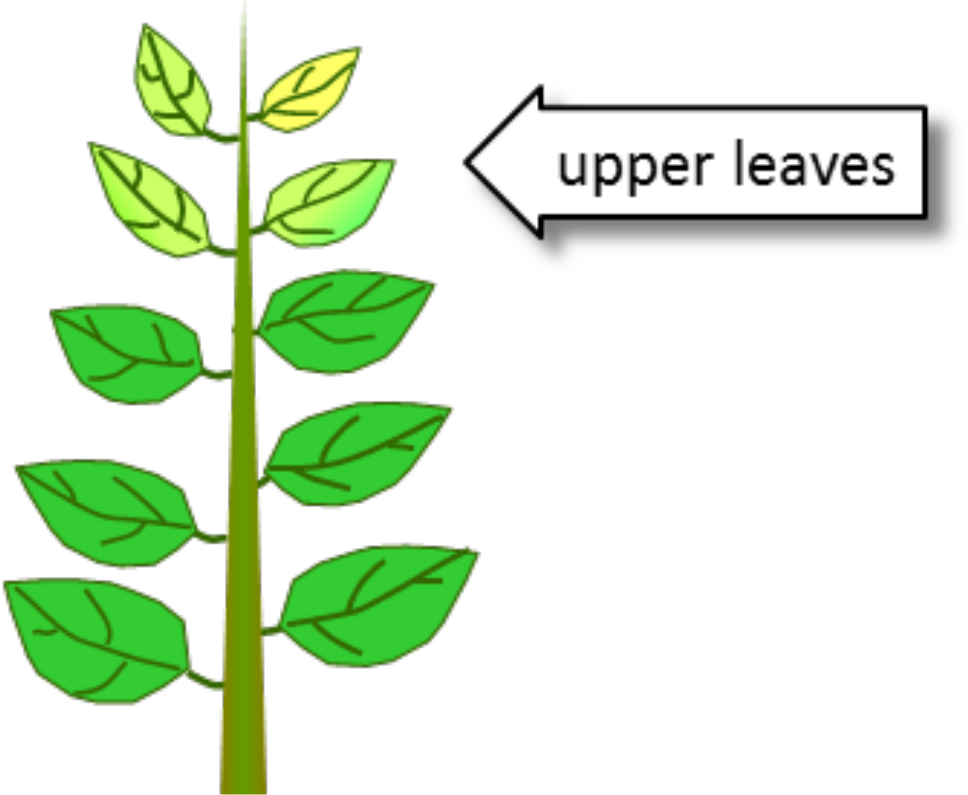
¶ C. Manganese Deficiency Conditions
- Deficiency occurs on high pH soils
- Mn2+ concentration in solution varies with pH
- Increased pH, decreased Mn2+ activity
- Liming very acid soils can precipitate Mn2+ as MnO2
- Deficiencies typically found in calcareous soils or can be induced by over-liming poorly buffered soils
- Mn2+ concentration in solution varies with pH
- Deficiency can occur on high organic matter soils, especially peats and mucks
- May combine with high molecular weight organic materials, like lignin
- Can form unavailable, chelated Mn2+ compounds
- Nutrient interactions
- High copper (Cu), iron (Fe), and zinc (Zn) can induce deficiency
- Acidifying effect of ammonium (NH4-N) fertilizers can increase manganese availability
- Conversion of ammonium (NH4+) to nitrate (NO3-) releases hydrogen ions (H+)
- Dry weather can induce deficiency on marginal soils
- Warm, dry conditions encourage formation of less available, oxidized forms; conversion from Mn2+
- Crop manganese requirements differ
- High requirement: beans (lima, snap), lettuce, oat, onion, radish, raspberry, soybean, spinach, sorghum-sudan, wheat
- Medium requirement: barley, beet, broccoli, brussels sprout, cabbage, carrot cauliflower, celery, corn, cucumber, pea, potato, tobacco, tomato
¶ Figure 2. Sources of Manganese
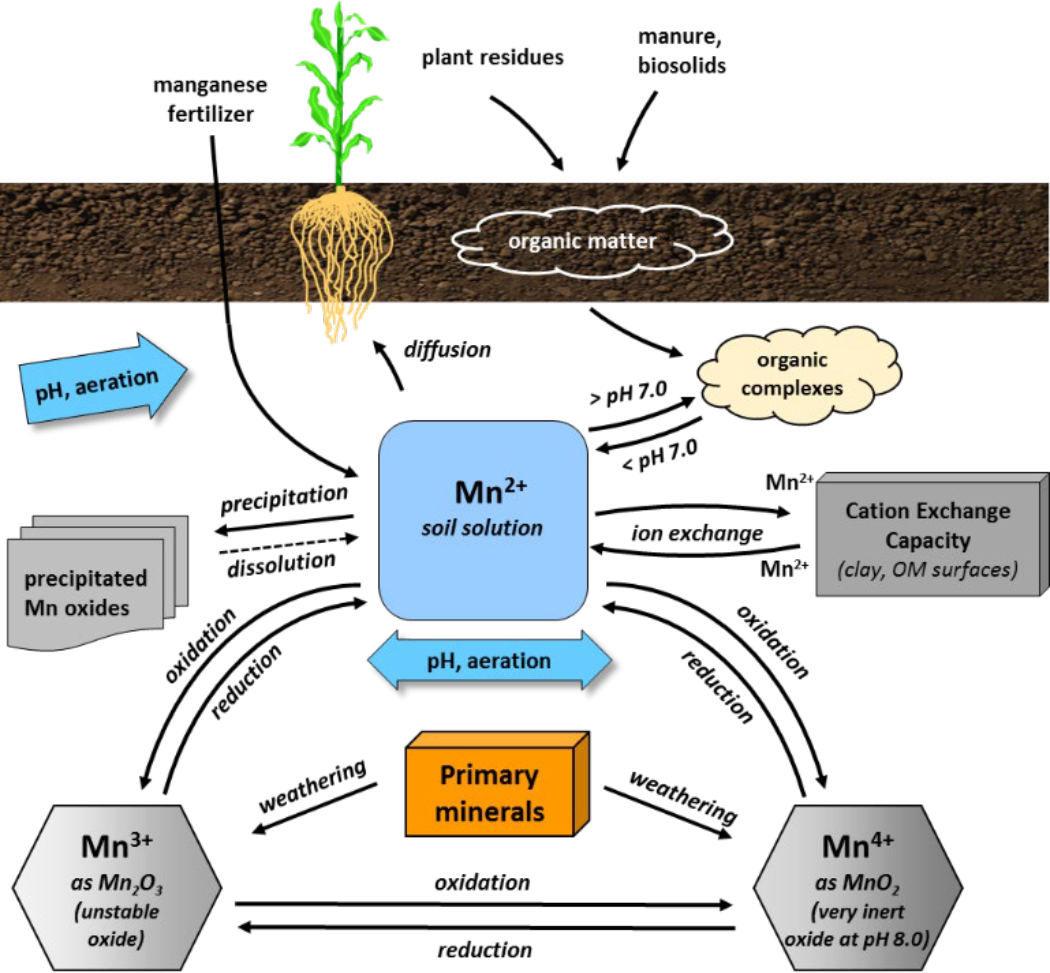
¶ D. Manganese Toxicity Conditions
- Toxicity can occur on very acid soils
- Occurs frequently in tandem with aluminum toxicity
- Manganese minerals become highly soluble, perhaps toxic, when pH drops below 5.2
- MnO2 + 4H+ + 2e- → Mn2+ + 2H2O
- MnO2 = solid manganese, not available to plants
- Mn2+ = soluble manganese, readily available for plant uptake
- e- = electrons
- Sources of electrons
- Often provided directly by organic amendments
- Animal manures, crop residues, composts
- Moist, organic, acidic soils are most susceptible to toxicity
- Toxicity may be induced indirectly by depriving soil of oxygen (O2)
- Typically occurs in poorly drained or flooded soils
- Liming usually eliminates toxicity
- Increasing soil pH causes manganese to precipitate as less soluble compounds; reduces the excess of available manganese
¶ E. Soil testing for Manganese
- Soil analysis levels for manganese not well calibrated with crop response
- Analytical results can be variable.
- Soil characteristics and plant species are better predictors of potential deficiency conditions.
- Deficiency symptoms usually reoccur in same area.
- Synthetic chelate extractants
- Act somewhat like organic chelating compounds that are exuded by plant roots.
- DTPA (diethylenetriaminepentaacetic acid).
- Better adapted for neutral to alkaline soils?
- Commonly used in Plains and Western states
- Common critical level: 2 to 4 ppm DTPA-Mn
- Determined by ICP or atomic absorption
- Acidic extractants
- Hydrochloric acid (0.1 M HCl) determined by ICP
- Generally used in Corn Belt states
- Critical level: 10 to 40 ppm Mn, depending on soil pH
- Mehlich-1 (determined by ICP)
- Includes two acids adjusted to pH 1.2.
- Hydrochloric acid (0.05 M HCl)
- Sulfuric acid (0.0125 M H2SO4)
- Generally used by states along southern Atlantic Coast.
- Critical range from about 3 to 9 ppm Mn
- Includes two acids adjusted to pH 1.2.
- Modified Morgan (determined by ICP)
- Includes acetic acid (0.52 N CH3COOH) adjusted to pH 4.8
- Used primarily in Northeastern states
- Critical ranges from 0.2 to 10.2 ppm Mn, depending on pH
- Phosphoric acid (0.033 M H3PO4)
- Used in Michigan, Ohio, Wisconsin.
- Critical ranges not identified
- Hydrochloric acid (0.1 M HCl) determined by ICP
- Combination extractants
- Mehlich-3 (determined by ICP)
- includes both chelate and acids adjusted to pH 2.5
- Ethylenediaminetetraacetic acid (0.001 M EDTA)
- Nitric acid (0.013 M HNO3)
- Acetic acid (0.2 N CH3COOH)
- Used in many states east of Rocky Mountains
- Common critical levels developed in Southeast states
- Depends on soil pH, soil aeration, and other factors.
- Critical level ranges from about 20 to 25 ppm Mn in neutral to alkaline soils; declines to about 3 to 5 ppm Mn in acidic soils.
- includes both chelate and acids adjusted to pH 2.5
- Mehlich-3 (determined by ICP)
¶ F. Manganese Nutrient Management
- Manure and other organic sources
- Manure supplies manganese, about 0.2 to 0.5 lb Mn per wet ton.
- Biosolids also supply complex organic molecules which help chelate manganese ions.
- Inorganic fertilizer materials
- Manganese sulfate, MnSO4● 4H2O
- Most common manganese fertilizer source
- Common analysis: 26% to 28% Mn
- 70% to 100% water soluble
- Manganese chloride, MnCl2
- Common analysis: 17% Mn
- 100% water soluble
- Manganese carbonate, MnCO3
- Common analysis: 31% Mn
- Manganese oxide, MnO
- Common analysis: 68 -70% Mn
- Insoluble in water
- Manganese nitrate, Mn(NO3)2
- Common analysis: <30% Mn
- 100% water soluble
- Manganese sulfate, MnSO4● 4H2O
- Organic, non-biological materials
- Synthetic chelates
- Mn-EDTA,
- EDTA, ethylenediaminetetraacetic acid
- Common analysis: 5% to 12% Mn
- Used for foliar applications
- Mn-EDTA,
- Organic complexes
- Common analysis: 5% to 10%
- Includes citric acid, lignosulfonates, etc.
- Some are industrial byproducts
- Less expensive than synthetic chelates, not as effective
- Often blended with small amount of synthetic chelate to reduce cost.
- Lignosulfonates may be combined with inorganic fertilizer.
- Synthetic chelates
- Soil application
- Broadcast applications not recommended on high pH or high organic matter soils
- Have capacity to precipitate and rapidly “fix” manganese
- Band applications are preferred
- Beneficial to combine manganese and ammonium-based fertilizer
- Conversion of ammonium to nitrate generates acidity, lowers soil pH in band
- Soil applied manganese chelates often ineffective
- Soil iron or calcium replaces manganese in chelate molecule.
- Can potentially aggravate deficiency.
- Broadcast applications not recommended on high pH or high organic matter soils
- Foliar application
- Inorganic and chelated sources appear equally effective
- Can be effective at correcting deficiencies
- May require multiple, repeated applications
- Use lowest effective rate to avoid foliar burn
- Manganese fertilizers tank-mixed with glyphosate may reduce effectiveness of glyphosate
- Loss of control depends on weed species
- If glyphosate applied first, apply manganese two to four days later
- If manganese applied first, apply glyphosate seven or more days later
- Use EDTA-chelated manganese if it must be tank-mixed with glyphosate
- Mixing sequence: water → ammonium sulfate → glyphosate → Mn-EDTA
- Applications through sprinkler irrigation not effective
¶ References
Rosen. 2008. SOIL 3416: Plant Nutrients in the Environment. Univ. of Minnesota. Lecture 13 outline accessed 1/15/2008
http://www.soils.umn.edu/academics/classes/soil3416/lecture13.htm
Soil-Plant Nutrient Cycling & Environmental Quality, spring 1998, Oklahoma State Univ., class publication.
Tisdale, Nelson, Beaton, Havlin. 1993. Soil Fertility and Fertilizers (5th ed.). MacMillan Publishing, New York. pg. 70-71, 332-337
Mills, Jones. 1996. Plant Analysis Handbook II. Micro Macro Pub., Athens, Georgia. pg. 47-49.
Kelling, Schulte, Walsh. 1999. Soil and Applied Manganese. Pub. A2526. Univ. of Wisconsin Coop. Ext. Serv., Madison, Wisconsin. 4 pg.
Hochmuth & Hanlon. 1995. IFAS Standardized Fertilization Recommendations for Vegetable Crops. Univ. of Florida Ext. Pub CIR152. 8 pg. 09Mar2020 https://nutrients.ifas.ufl.edu/nutrient_pages/BSFpages/PIndices/CV00200.pdf
Heckman. 2000. Manganese Needs of Soils and Crops in New Jersey. Rutgers Coop. Ext. Pub. FS973. 4 pg.
Nathan, et.al. 2005. Evaluating Mehlich III Extractant for Extracting Available Nutrients for Missouri Soils using Inductively Coupled Plasma Spectrometry. Univ. of Missouri. 9 pg. http://aes.missouri.edu/pfcs/research/prop304a.pdf
Esipinosa, Slaton, Mozaffari. Understanding the Numbers on Your Soil Test Report. Univ. of Arkansas. Coop Ext. Svc. Pub. FSA2118. 4 pg. http://www.washingtonccd.org/uploads/6/9/1/2/6912341/understanding_soil_test.pdf
Camberato. 2004. Soil Fertility Series #1: Manganese Deficiency and Fertilization of Cotton. Clemson Univ. Ext. Svc. 4 pg.
Stanton. 2016. Identifying and correcting manganese deficiency in soybeans. Michigan State Univ. Ext. 23June2016. http://msue.anr.msu.edu/news/identifying_and_correcting_manganese_deficiency_in_soybeans