⇦ Back to Soil Fertility and Plant Nutrition Home
¶ A. Iron in the Plant
- Considered an essential micronutrient; required by plants in very small amounts
- Typical crop removals range from 0.05 to 1.65 lb/ac Fe
- Content in most plant tissues between 50 to 75 ppm Fe; commonly range from 20 to 300 ppm Fe
- Ferric form of iron is dominant form in soil, but ferrous form of iron is physiologically active
- Ferric iron, Fe3+ or oxidized iron or “iron(III)”
- Ferrous iron, Fe2+ or reduced iron or “iron(II)”
- Ferric Fe3+ is predominant state in aqueous, non-acidic, oxygenated environments
- Can be taken up by plant roots as ferric iron or ferrous iron
- Most readily taken up as Fe2+ (ferrous iron)
- Fe3+ (ferric iron) is generally reduced to Fe2+ before actual absorption occurs
- Fe3+ uptake important for grasses
- Not readily translocated in plant
- Transported in xylem mainly to chloroplast
- Deficiencies occur first in growing points, young leaves
- Involved in oxidation-reduction (“redox”) reactions
- Chlorophyll synthesis
- Component of cytochromes, ferredoxin,
- Component of leghemoglobin
- Gives pink color to interior of effective legume nodules
- Necessary for photosynthesis, respiration, nitrogen fixation
- Plants differ in iron-uptake efficiency
- Is unique micronutrient because uptake and availability can be influenced by plant itself
- Wide range of ability to take up adequate iron with low levels of available iron in soil solution
- Are Fe-efficient vs. Fe-inefficient plants
- Uptake and nutrition are genetically controlled
- Are differences between plant species
- Are differences between crop genotypes, hybrids, varieties, within species
- Roots of iron-efficient crops make adaptive responses to low iron in soil solution
- Several mechanisms have been identified in root tips and root hairs, but are not fully understood
- Increase rate of iron absorption permitting more rapid Fe3+ reduction
- Improve iron translocation from roots to shoots
- Formation of transfer cells
- Citrate and other organic acids transport iron
- Graminaceous monocots (grasses, cereals, rice) utilize common strategy
- Roots secrete phytosiderophores into rhizosphere
- Are plant synthesized molecules
- Act as chelating agents to transport Fe3+ (ferrous iron) across cell membrane
- Roots secrete phytosiderophores into rhizosphere
- Dicots and non-graminaceous monocots utilize common strategies
- Release H+ to acidify rhizhosphere
- Solubilize ferric iron
- Exude phenolic compounds in low iron situations
- Phenolics may directly solubilize and reduce iron in rhizosphere
- Phenolics can chelate iron and remobilize iron sorbed onto cell walls
- Release H+ to acidify rhizhosphere
- Several mechanisms have been identified in root tips and root hairs, but are not fully understood
- Deficiency symptoms
- Interveinal chlorosis is primary symptom
- May appear similar to manganese, magnesium, or potassium deficiency
- Iron and manganese not mobile, so upper or younger leaves affected
- Magnesium and potassium are mobile, so older, lower leaves affected
- Leaves may turn white and dry up in severe deficiency
- Chlorosis may spread to older leaves
- Leaf tissue levels are often very high in chlorotic plants; may be comparatively low in non-chlorotic plants
- Opposite of common expectations (i.e., low = deficient; high = adequate)
- Protein (total nitrogen) and nitrate tend to accumulate during deficiency
¶ Figure 1. Iron Deficiencies
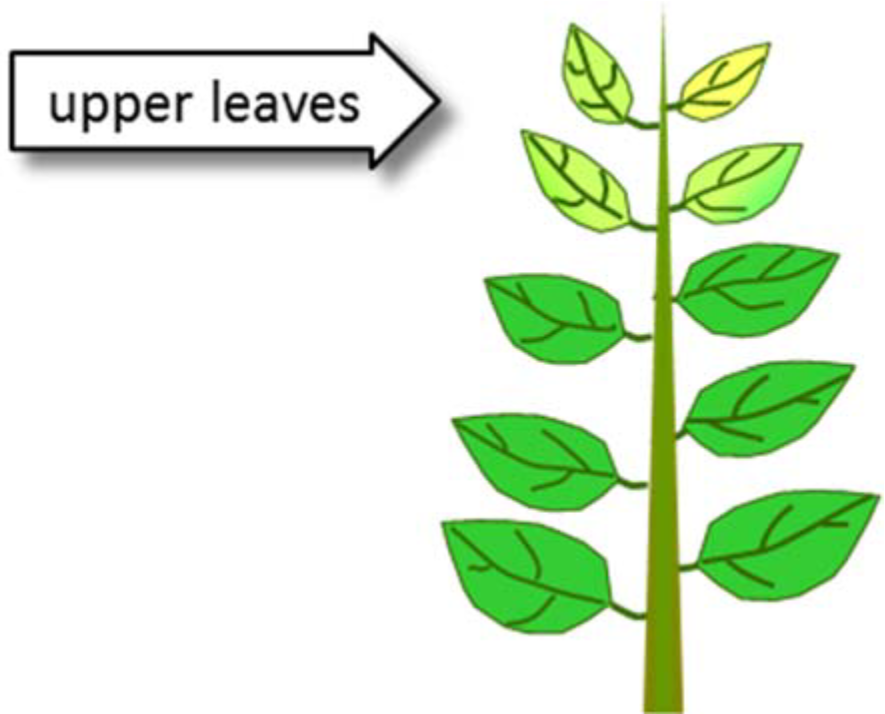
¶ B. Iron in the Soil
- Iron chemistry very complicated; depends largely on pH and redox potential
- Oxidation: Fe2+ + ¼O2 + H+ → Fe3+ + ½ H2O
- Reduction: Fe3+ + e- → Fe2+
- Iron minerals
- Very abundant in earth's crust; are about 5% of total soil chemical composition
- Primary iron minerals include clays, oxides, hydroxides
- Ferrous minerals
- Pyrite (FeS2)
- Magnetite, Fe3O4
- Hydrolysis products, FeOH+, Fe(OH)20
- Ferric minerals
- Iron hydroxide, Fe(OH)3
- ii. Geothite, FeOOH
- iii. Hematite, Fe2O3
- iv. Jarosite, KFe3(SO4)2(OH)6
- Bacterial oxidation of pyrite generates acid; common cause of acid mine drainage
- FeS2 + 3½O2 + H2O → Fe2+ + 2SO42- J+ 2H+
- Soil solution iron
- Solubility of iron minerals very low
- Amorphous, unstructured Fe(OH)3 minerals usually most soluble form of iron in soil
- Typically controls iron solubility in aerobic soils
- Well-drained, oxidized soils
- Fe3+ concentrations much higher than Fe2+
- Soils have reddish color due to oxidized iron
- Water-saturated soils
- Ferrous iron is reduced to ferric iron, Fe3+ → Fe2+
- Soils have gray color due to reduced iron
- Solubility of iron minerals very low
- Iron solubility and pH
- Iron deficiencies most common in high pH soils
- Fe(OH)3 (soil) + 3H+ ↔ Fe3+ + 3H2O
- Soluble ferric iron ion (Fe3+) decreases 1000 times with each 1 unit that soil pH increases
- Iron moves to roots by both diffusion and mass flow, but slowly compared to other nutrients
- Soil solution concentrations very low
- Fe3+ ion concentration ≈ 10-6 to 10-24 M
- Total iron in solution is too low to meet crop needs
- Even in acidic soils with highest iron concentrations
- Chelation is required to supply adequate iron to plant roots
- Increases amount of iron in soluble form
- Increases quantity of iron moved by diffusion and mass flow
- Soil solution concentrations very low
¶ Figure 2. Iron Sources
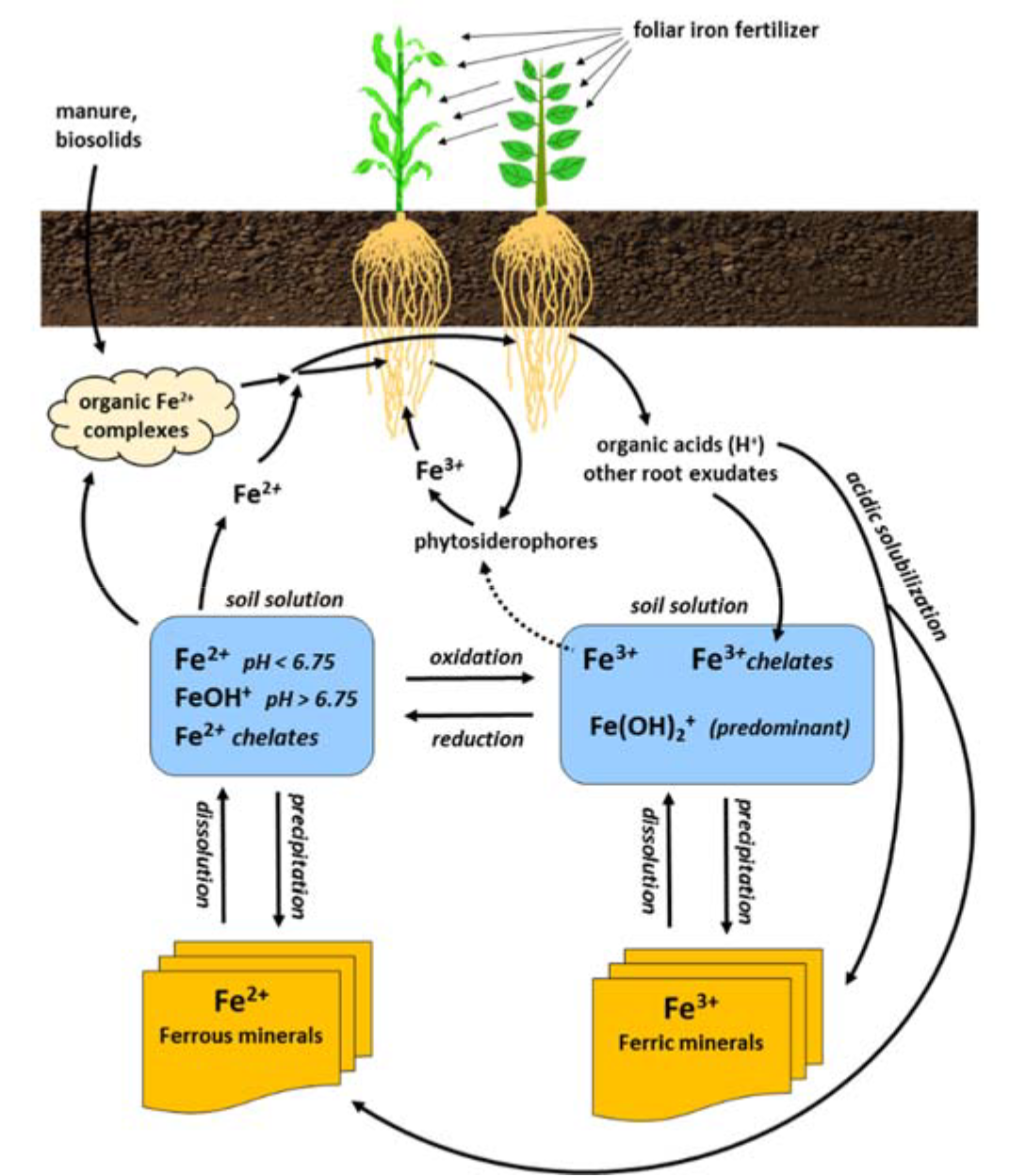
¶ C. Chelation Process
- “Chelation” is complexation of metal ions by organic molecules that may originate from:
- Compounds synthesized by roots
- Degradation of soil organic and plant residues
- Products of microbial metabolism
- Natural chelates (including citric and oxalic acids)
- Structure of many natural chelates is unknown
- Word ”chelate” is derived from Latin word for "claw"
- Chelate molecule has multiple binding sites for metals, like iron
- Chelate "surrounds" the metal ion
- Soluble chelates increase metal availability by protecting them from precipitation/adsorption reactions
- In some cases (e.g., copper), chelation by functional groups of solid organic matter can reduce availability
- Chelation and iron uptake process
- Step 1: Chelation removes free iron from solution
- Step 2: Chelated iron diffuses to plant roots
- Step 3: Fe3+ ion released at root surface by chelate compound
- Step 4: Free chelate compound diffuses back to the bulk solution
- Step 5: Chelate complexes with another Fe3+ ion
- Step 6: repeat step 1
- As chelation removes free iron from solution, iron concentration in soil solution decreases.
- Depletion of soil solution iron causes release of adsorbed iron or dissolution of iron minerals to replenish iron in soil solution
¶ D. Iron Deficiency Conditions
- Lime-induced iron deficiency chlorosis
- High pH, calcareous soils
- Have free carbonates
- Soil test rating of “HI” excess lime
- Often poorly drained soils with poor aeration
- Fine carbonates neutralize acids secreted by plant roots that were meant to solubilize soil iron
- Soil pH > 7.5 often affects soybean, sorghum, pin oak
- Soil pH > 5.5 often affects azaleas and blueberries
- Erosion or land leveling may expose subsoil that contains free carbonates
- High pH, calcareous soils
- Wet soils aggravate iron chlorosis
- Limited air exchange in soil; allows buildup of carbon dioxide from respiration of roots and microbes
- Results in high bicarbonate in soil solution
- Especially if free carbonates are present
- Severity greater with low temperatures
- Nitrate vs. ammonium
- Acidifying effect of nitrification
- Conversion of NH4+ to NO3- releases H+
- Rhizosphere acidification with NH4-N uptake
- Also affects availability and uptake of other micronutrients
- Acidifying effect of nitrification
- High soil nitrates can aggravate chlorosis
- High nitrate effect documented in soybeans
- Plant root must exchange bicarbonate ion into soil solution to take up nitrate ion
- Nitrate must be converted to ammonium within leaves
- Increases pH of leaf sap
- Changing pH slows rate of reducing reaction(s) that convert Fe3+ to Fe2+ (ferric to ferrous)
- Wheel tracks through chlorotic area may be green
- Some soil compaction may result from wheel traffic causing lower porosity and slower water percolation
- Soil under wheel tracks may stay wet for longer than adjacent soil
- Wet conditions may cause some denitrification of nitrate (NO3-) to atmospheric nitrogen (N2)
- Lower nitrate, less need for roots to exchange bicarbonate
- Low organic matter
- Erosion or land leveling may expose subsoil with lower organic matter content
- Lack of chelating compounds (organic complexes) can be more important than lack of iron
- Nutrient interactions
- Excess copper, manganese, zinc, molybdenum, phosphorus can induce deficiency
¶ E. Toxicity Conditions
- Occurs in very poorly drained conditions, especially acidic soils
- Common problem in paddy rice
- Excess soluble Fe2+ produced in reducing environment
- Roots are scanty, coarse, and often dark brown due to coatings of ferric oxide.
¶ F. Soil Testing for Iron
- Soil analysis levels for iron poorly calibrated with crop response
- Analytical results can be highly variable
- Soil characteristics and plant species are better predictors of deficiency conditions
- Synthetic chelates used for extraction
- Act somewhat like organic compounds exuded by root
- DTPA (diethylene-triamine-pentaacetic acid), C14H23O10N3
- Used alone as extractant for micronutrient metals
- Component of ammonium-bicarbonate-DTPA multi-element extractant
- Common critical level: 2.5 to 5.0 DTPA-Fe
- EDTA (ethylene-diamine-tetraacetic acid), C10H16O6N2
- Component of Mehlich-3 multi-element extractant
¶ G. Iron Nutrient Management
- Application
- Soil applications generally not effective
- Problem is iron availability, not amount applied
- Foliar applications generally most effective
- Iron-sulfates or synthetic chelates
- Ortho-ortho EDDHA chelates effective in soil
- Soil applications generally not effective
- Organic, biological sources
- Manure supplies iron
- Content variable, ranges from < 1 to 15 lb/ton
- Biosolids also supply organic molecules that act as chelates to maintain iron availability
- Single application (e.g., 15 to 20 tons of stockpiled manure) may effectively treat chlorosis for two to three years
- Manure supplies iron
- Inorganic fertilizer materials
- Ferrous sulfate heptahydrate
- FeSO4.•7H2O
- Common analysis: 19% to 20% Fe, 12% S
- Also known as “copperas”; has blue-green color
- Ferrous ammonium sulfate,
- (NH4)2SO4•FeSO4•6H2O
- Common analysis: 14% Fe, 10% N, 21% S
- Blend of iron (2+) sulfate monohydrate and ammonium sulfate
- Also known as “Mohr’s salt”
- Ferrous sulfate heptahydrate
- Organic, non-biological materials
- Synthetic chelates
- Can be both soil and foliar applied
- EDTA, 9% - 12% Fe
- EDDHA, ethylenediaminedi(o-hydroxyphenyl-acetic) acid
- Common analysis: 6% Fe
- Iron remains complexed over broad pH range
- Organic complexes
- Includes citric acid (C6H8O7), lignosulfonates, polyflavinoids, etc.
- Typically 5% to 10% Fe
- Some are industrial byproducts
- Not as effective as synthetic chelates, but less expensive
- Often blended with small amount of synthetic chelate
- Includes citric acid (C6H8O7), lignosulfonates, polyflavinoids, etc.
- Soil applied chelated iron
- Calcium replaces iron in most synthetic chelates, except EDDHA, in neutral and calcareous soils
- Can be effective in some situations, but are expensive
- Often limited to high-value crops
- Synthetic chelates
¶ H. Iron Deficiency Chlorosis (IDC) Management
- Select chlorosis-tolerant varieties for IDC-prone areas
- Yield potential of chlorosis-tolerant varieties may be different than chlorosis-susceptible varieties
- Example: small-seeded edible bean varieties (black and navy) are more susceptible than medium-sized seeds (pinto, great northern, pink, and small reds)
- Areas with IDC potential are often small and intermittent
- Variable rate planting?
- Include other treatment methods to improve overall effectiveness
- Yield potential of chlorosis-tolerant varieties may be different than chlorosis-susceptible varieties
- Plant companion crop (e.g., oats)
- Removes excess nitrate
- Helps dry out soil, reducing bicarbonate build up
- Must be managed carefully to limit yield
- Proper termination timing, etc.
- Minimize plant stress
- Limit potential for soil compaction problems
- Consider stress potential of soil-applied herbicides, fertilizer placement, etc.
- Seed-placed iron product
- Most iron fertilizers ineffective
- Iron salts rapidly precipitate as Fe(OH)3
- Calcium displaces iron from most synthetic chelates in neutral and calcareous soils
- Per Nebraska research: at-planting seed-row application of 50 to 100 pounds of ferrous sulfate heptahydrate has proven effective in corn
- Requires dry fertilizer equipment on planter
- High per-acre product cost
- Ortho-ortho EDDHA chelated iron proven effective
- EDDHA = ethylenediamine-N,N'-bis(2-hydroxyphenylacetic acid)
- Example: 6% EDDHA chelate product
- “ortho” and “para” refer to different chemical structures
- Older ortho-para chelate products not effective
- Some products are blend of ortho-para and ortho-ortho chelates
- Lower ortho-ortho content reduces effectiveness
- Most iron fertilizers ineffective
- Foliar sprays
- Not easy to predict when response will occur
- Small areas with IDC potential are often scattered within field
- IDC severity affected by short-term weather conditions
- Spot treatments are warranted, but may not be practical in all situations
- Repeated or multiple applications often required
- Good leaf coverage essential (fine droplets, high carrier rate)
- Sprinkler irrigation applications of iron fertilizers not effective
- Iron sulfate spray mixture
- Ferrous sulfate solution
- 16 lb. ferrous sulfate (19% to 20% Fe; may be known as “copperas” fertilizer)
- 100 gallons water
- 8.3 gallons UAN solution (28-0-0)
- Application volume
- Ground application: 20 to 40 gal/ac
- Aerial application: 5 gal/ac minimum
- Solution may develop less soluble, yellowish oxides when exposed to air
- Oxides may plug nozzles
- Premix solution and strain before filling applicator to remove particulates
- Do not use other “iron sulfate” products
- e.g., ferrous sulfate monohydrate, 30% Fe
- Monohydrate ferrous sulfate has lower water solubility than heptahydrate ferrous sulfate
- Is grayish color product
- Ferrous sulfate solution
- Not easy to predict when response will occur
¶ References
Rosen. 2008. SOIL 3416: Plant Nutrients in the Environment. Univ. of Minnesota. Lecture 13 outline accessed 1/15/2008
http://www.soils.umn.edu/academics/classes/soil3416/lecture13.htm
Soil-Plant Nutrient Cycling & Environmental Quality, spring 1998, Oklahoma State Univ., class publication.
Kaiser, Lamb, Bloom. 2011. Managing Iron Deficiency Chlorosis in Soybean. AG-FO-08672-A. Univ. of Minnesota Coop Ext. Serv. 4 pg.
Schulte. 1992. Soil and Applied Iron. Pub. A3544. Univ. of Wisconsin Coop. Ext. Serv., Madison, Wisconsin. 2 pg.
Jin, et. al. 2008. The iron deficiency-induced phenolics secretion plays multiple important roles in plant iron acquisition underground. Plant Signaling & Behavior. 3:60-61.
Tisdale, Nelson, Beaton, Havlin. 1993. Soil Fertility and Fertilizers (5th ed.). MacMillan Publishing, New York. pg. 67-70, 304-319
Mills, Jones. 1996. Plant Analysis Handbook II. Micro Maro Pub., Athens, Georgia. pg. 44-47.
Davis & Westfall. Fact Sheet No. 0.538. Fertilizing Corn. accessed 07/14/2016. http://extension.colostate.edu/topic-areas/agriculture/fertilizing-corn-0-538/
Moore, et.al. 2012. CIS 1189, Southern Idaho Fertilizer Guide: Beans. Univ. of Idaho Extension, Moscow ID. pg. 5.