⇦ Back to Soil Fertility and Plant Nutrition Home
¶ Zinc in the Plant
- Considered an essential micronutrient; required by plants in very small amounts
- Typical crop removals range from 0.1 to 0.5 lb/ac
(See Crop File 1.02.034, Nutrient removal values: Sulfur, Zinc) - Total zinc content in most plants ranges between 25 to 150 ppm Zn
- Common sufficiency range in leaves between 15 to 50 ppm Zn
- Typical crop removals range from 0.1 to 0.5 lb/ac
- Taken up by plant roots as zinc ion, Zn2+
- Primarily involved in enzyme activity
- Structural, functional, or regulatory cofactor
- Carbohydrate metabolism
- Protein synthesis
- Tryptophan and plant growth regulators
- Auxins, like IAA (indole-3-acetic acid)
- Not readily translocated in plant
- Deficiencies often occur first in growing points, young leaves
- In some plant species, deficiency symptoms may occur on older, lower leaves
- Deficiency symptoms
- Stunted growth
- Shortened internodes, “rosette”, bushy appearance
- rosette = circular arrangement of leaves, with all leaves at similar height
- Caused by lack of IAA (auxin that induces cell elongation)
- Shortened internodes, “rosette”, bushy appearance
- Light green, yellow, or white discoloration of leaves
- Chlorotic bands along midribs of monocot leaves, especially corn
- Yellowing, bronzing of lower leaves of beans
- Small, narrow, thickened leaves; “little leaf” syndrome
- Defoliation
- Malformed fruit
- Stunted growth
¶
Figure 1. Deficiencies of Zinc
¶ B. Zinc in the Soil
- Contained in small amounts in certain primary and secondary soil minerals
- Total soil content ranges from 10 to 300 ppm Zn; averages about 50 ppm Zn
- Total zinc concentration increases with depth
- Extractable zinc content decreases with depth
- Organic matter level affects zinc concentration in surface soil and subsoils
- Root uptake
- Zinc ion, Zn2+, moves primarily by diffusion
- Some movement by mass flow
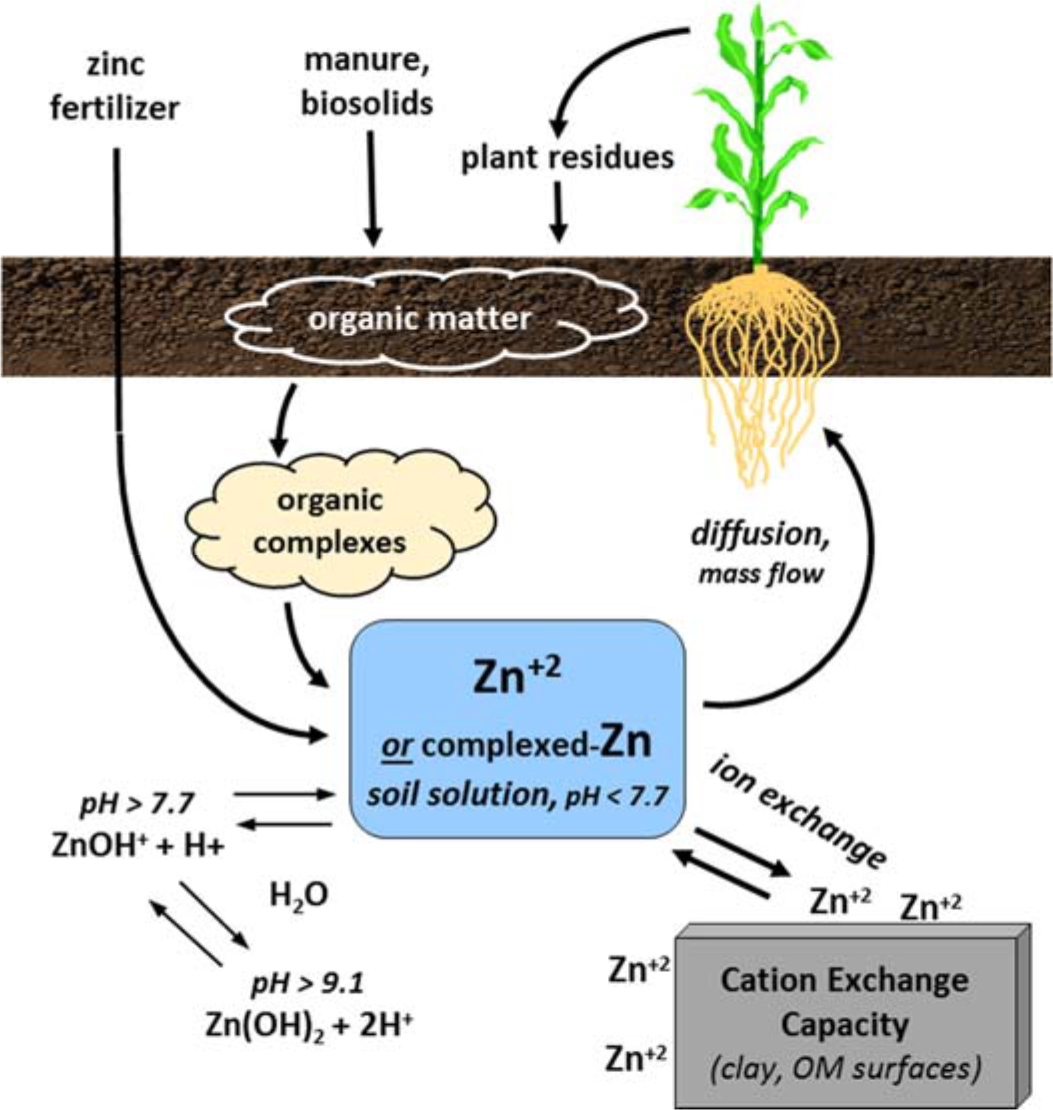
- Soil solution zinc
- Zn solubility primarily controlled by solution pH and adsorption on mineral and organic surfaces
- Soil-Zn (mineral zinc) + 2H+ ↔ Zn2+
- Soluble zinc ion (Zn2+) decreases 100-fold for each 1 unit increase in pH
- Zinc adsorption increases with increasing soil pH
- pH is most important factor affecting zinc solubility and mobility
- Zn2+ is predominant form at pH < 7.7
- ii. ZnOH+ is predominant form at pH > 7.7
- iii. Zn(OH)2 is predominant form at pH > 9.1
- Majority of solution zinc is often found in “chelated” forms (i.e., organic complexes)
- Diffusion of chelated zinc important for adequate supply to plant roots
- Zn solubility primarily controlled by solution pH and adsorption on mineral and organic surfaces
- Zinc adsorption
- Zinc adsorbed on clay, aluminum-oxide, iron-oxide, organic matter, and carbonate surfaces
- These surfaces have wide range of adsorption strengths
- Readily exchangeable to very strongly bound
- Organic matter complexes both increases and decrease zinc availability
- Adsorption to exchange surfaces of insoluble organic matter decreases solution zinc
- Solubilization and mobilization by short-chain organic compounds
- Immobilization by high molecular weight organic materials, like lignin
- Initially complexed zinc may then form insoluble salts
- Organic chelation
- Complexation of metal ions by organic molecules
- Includes organic compounds synthesized by roots and products of microbial metabolism
- e.g. organic acids, organic bases
- Chelate compound "surrounds" zinc ion
- Typically increases amount of zinc in soluble form increasing availability to root system
- Can temporarily protect zinc cation from combining with other anions and forming insoluble compounds
- Increases quantity of zinc moved by diffusion and mass flow to root surface
- Includes organic compounds synthesized by roots and products of microbial metabolism
- Complexation of metal ions by organic molecules
¶ Figure 2. Sources of Zinc
¶ C. Zinc Deficiency Conditions
- High pH, calcareous soils
- Deficiency common in eroded slopes, areas with land leveling
- Topsoil is removed, exposing high clay, calcareous subsoil materials
- Organic matter removed with topsoil
- Deficiency common in eroded slopes, areas with land leveling
- Fine-textured soils
- High adsorption capacity from large surface area due to higher clay content
- Cool, wet soil conditions
- Slower root growth and development
- Slower chemical reactions
- Early spring deficiencies may occur, regardless of soil test zinc level
- Deficiency symptoms may disappear with warmer temperatures (transient deficiency)
- Anaerobic and reducing conditions in flooded calcareous soils may increase pH and reduce zinc solubility
- Nutrient interactions
- Ammonium (NH4+) vs. nitrate (NO3-)
- Acidification resulting from nitrification can affect Zn2+ activity
- Zinc-phosphorus interaction
- Not common occurrence
- High soil phosphorus, high phosphate fertilizer applications, marginal to low soil zinc
- Tissue P-to-Zn ratios in excess of 100:1
- May be characteristic response of certain hybrid or inbred line
- Excess copper, iron, and manganese can induce deficiency
- Ammonium (NH4+) vs. nitrate (NO3-)
- Crop sensitivity to low soil levels
- Sensitive: corn, field beans, sweet corn, peanuts (at low pH)
- Moderately tolerant: grain sorghum, clover, potatoes, forage sorghum, soybeans, sugarbeets, sudangrass
- Tolerant: alfalfa, barley, oats, millet, rye, wheat, grasses
- Increased likelihood of deficiency in sensitive crops that follow sugarbeet crop
¶ D. Zinc Excess Conditions
- Excess zinc symptoms
- Levels above 100 to 200 ppm Zn in leaves
- Reduced root growth and leaf expansion, followed by chlorosis
- Induced deficiency of iron and/or magnesium
- Sensitivity to zinc toxicity varies
- Sensitivity to zinc toxicity: microorganisms > soil fauna (earthworms, etc.) > plants
- Excess soil zinc usually caused by pollution due to high applications of zinc-rich waste materials
- Usually not problem in normal agricultural soils, application rates regulated by environmental agencies
- Soil concentration of 200 to 500 ppm total zinc could affect yields
- If soils are zinc contaminated, typically contaminated by other heavy metals (e.g., cadmium, lead, etc.)
- Major concern is with food crops for direct human consumption (vegetables, etc.) or feedstuffs fed to dairy animals
¶ E. Soil Testing for Zinc
- Synthetic chelates are used as extractant
- Act somewhat like organic chelating compounds exuded by root
- DTPA (diethylenetriaminepentaacetic acid)
- Better adapted for high pH and calcareous soils
- Typically used in Plains and Western states
- Common critical levels:
- Sensitive crops (corn, sorghum, beans): 0.8 to 1.2 ppm DTPA-Zn
- Less sensitive crops (grasses, small grains): 0.2 to 0.3 ppm DTPA-Zn
- Highly sensitive crops (pecans): less than 2.0 ppm DTPA-Zn
- EDTA (ethylenediaminetetraacetic acid)
- Is component of Mehlich-3 extracting solution
- Used in Southeast and Eastern U.S.
- Common critical level for row crops, field crops, truck crops: 2.0 to 2.5 ppm Mehlich-3 Zn
- Acid used as extractant
- Hydrochloric acid (0.1 M HCl)
- Used in Midwest Corn Belt states (North Central region) states
- Interpretation depends on soil pH, excess lime (reactive carbonates); possibly other soil properties
- Hydrochloric acid (0.1 M HCl)
¶ F. Zinc Nutrient Management
- Zinc ion immobile in soil
- All sources can correct deficiency in long-term, but placement relative to root system is critical in short-term
- Must be soil incorporated or applied in subsurface band to be effective
- Little agronomic difference between sources when applied at same rate of actual zinc and using same method
- Increased water solubility of product linked to increased effectiveness
- Organic, biological sources
- Manure and other organic sources may contain zinc
- Manure supplies about 0.1 to 0.5 lb Zn/wet ton, depending on manure source
- Biosolids also supply complex organic molecules which help complex or chelate zinc ions which help improve availability
- Manure and other organic sources may contain zinc
- Inorganic fertilizer materials
- Zinc sulfate, ZnSO4
- Common analysis: 22% to 36% Zn, 13% to 17% S
- May be by-product of galvanizing process
- About 98% water soluble
- Usually marketed in granular form
- Zinc oxide, ZnO
- Common analysis: 50% to 80% Zn
- Nearly insoluble; must be finely ground to be effective
- More effective in acid soils
- May be used in liquid suspension fertilizers
- Zinc oxysulfate, xZnSO4•xZnO
- Common analysis: 20% to 50% Zn
- Variable water solubility; depends on proportion of ZnSO4 or ZnO in product
- Produced by acidifying ZnO with sulfuric acid (H2SO4)
- Usually marketed in granular form
- Zinc-ammonium complex
- Also known as “ammoniated zinc”
- Common analysis: 9% to 10% Zn, 10% N; also available with 15% to 20% Zn
- Certain amount can be mixed with ammonium polyphosphate (10-34-0); zinc ions occupy sequestering sites and remain in solution
- Zinc sulfate, ZnSO4
- Organic, non-biological fertilizer materials
- Synthetic chelates
- EDTA commonly used for chelated zinc fertilizer
- Used for both soil and foliar application
- Can be effective as post-plant rescue treatment under deficiency conditions
- Typically most expensive source
- Organic complexes
- Not as effective as synthetic chelates, but less expensive; performance similar to zinc sulfate
- Often blended with small amount of synthetic chelate
- Some are industrial byproducts
- Citric acid, other organic acids
- Lignosulfonates -from paper industry
- Common analysis: 10% Zn, 7% S
- Highly water soluble
- Sucrates - from sugar industry
- Less than 1% water solubility
- Synthetic chelates
- Broadcast applications
- Typical recommendation is for single broadcast application rate to build soil test to critical level
- Single application considered effective for about three to five years
- Requires thorough soil incorporation
- Water solubility important in short term
- Little to no agronomic difference between sources applied at same rate and method in longer term
- Uniformity of distribution across soil surface is important
- Typical recommendation is for single broadcast application rate to build soil test to critical level
- Banded applications
- At-planting or starter applications often more effective in short-term than broadcast applications at same rates
- Banding most useful with low soil test levels, sensitive crops, and/or cold soils at planting
- Banding annually at reduced rates can maintain soil test level
- Chelates often claim higher effectiveness (10:1, 5:1, etc.) when compared to inorganic zinc sources, so zinc application rates reduced accordingly
- Research shows essentially no agronomic difference when products are applied at same rate and same method.
- Drastically reduced rates often result in long-term decline of zinc soil test
- Zinc fertilizer products generally do not contribute to fertilizer “salt” injury
- Foliar applications
- Common practice with orchard crops
- Stone fruits (peaches, etc.)
- Pomes (apples, pears)
- Nuts (pecans, walnuts, etc.)
- Can help with recovery of deficient seedlings
- Common practice with orchard crops
¶ References
Rosen. 2008. SOIL 3416: Plant Nutrients in the Environment. Univ. of Minnesota. Lecture 13 outline accessed 1/15/2008
http://www.soils.umn.edu/academics/classes/soil3416/lecture13.htm
Soil-Plant Nutrient Cycling & Environmental Quality, spring 1998, Oklahoma State Univ., class publication.
Tisdale, Nelson, Beaton, Havlin. 1993. Soil Fertility and Fertilizers (5th ed.). MacMillan Publishing, New York. pg. 71-72, 319-326
Mills, Jones. 1996. Plant Analysis Handbook II. Micro Maro Pub., Athens, Georgia. pg. 51-54.
Kelling, Schulte, Walsh. 1999. Soil and Applied Copper. Pub. A2522. Univ. of Wisconsin Coop. Ext. Serv., Madison, Wisconsin. 2 pg.
Shaver. 2014. “Micronutrients” in Nutrient Management for Agronomic Crop sin Nebraska, Pub #EC-155. Univ. of Nebraska, Coop Ext. Serv. pg 47-49
Intl Zinc Assn. accessed Apr 2016. Zinc in Fertilizer: options to consider
http://www.zinc.org/wp-content/uploads/sites/4/2015/04/Zinc-in-Fertilizer-options-to-consider.pdf