⇦ Back to Soil Fertility and Plant Nutrition Home
¶ Introduction
Sulfur is an essential element, a constituent of many proteins and metabolic cofactors. The sulfur cycle is the collection of processes by which sulfur moves to and from minerals and living systems.
Minerals like as pyrite (FeS2) were the original pool of sulfur on earth. Over time, these minerals have been converted and continue to be converted to various forms of sulfur. Crop File 1.04.550, “Sulfur in the Soil and Plant” discussed general roles and reactions of sulfur. The following Crop File looks at the major soil sulfur transformations in more detail
¶ A. Mineralization ↔ immobilization
- Organic sulfur cycling is similar to organic nitrogen cycling
- Affected significantly by microbial activity, temperature, and soil moisture
- Mineralization
- Release of organic sulfur into inorganic forms, including:
- Sulfate (SO4-2,),“plant-available”
- Hydrogen sulfide (H2S)
- Elemental sulfur
- Sulfide minerals
- Organic matter decomposition
- Affected by activity of bacteria, fungi, and actinomycetes
- Activity influenced by temperature, moisture, aeration, pH, nutrient content, residue incorporation, particle size, surface area, etc.
- Sulfatase enzymes
- Similar to phosphatase enzymes
- Release sulfate from sulfate esters
- Release of organic sulfur into inorganic forms, including:
- Immobilization
- Reverse of mineralization
- Uptake of inorganic sulfur from the soil and incorporation into organic sulfur forms by microorganisms (microbial assimilation)
- Sulfur assimilation in which sulfate (SO42−) is reduced by plants, fungi and various prokaryotes to sulfhydryl groups (R-SH)
- Sulfate oxidation state is +6
- Sulfhydryl group oxidation state is -2
- R-group = abbreviation for any compound in which carbon or hydrogen atom is attached to rest of the molecule
- Balance between mineralization and immobilization is affected by carbon-to-sulfur (C:S) and nitrogen-to-sulfur (N:S) ratios of soil organic matter
- Soil organic matter carbon-to-nitrogen-to-sulfur (C:N:S) ratio approximately 120:10:1.4
- C:S ratio similar to carbon-to-phosphorus (C:P) ratio
- When C:S ratio greater than 400
- Immobilization > mineralization
- When C:S ratio = 200 to 400
- Immobilization = mineralization
- When C:S ratio less than 200
- Mineralization > immobilization
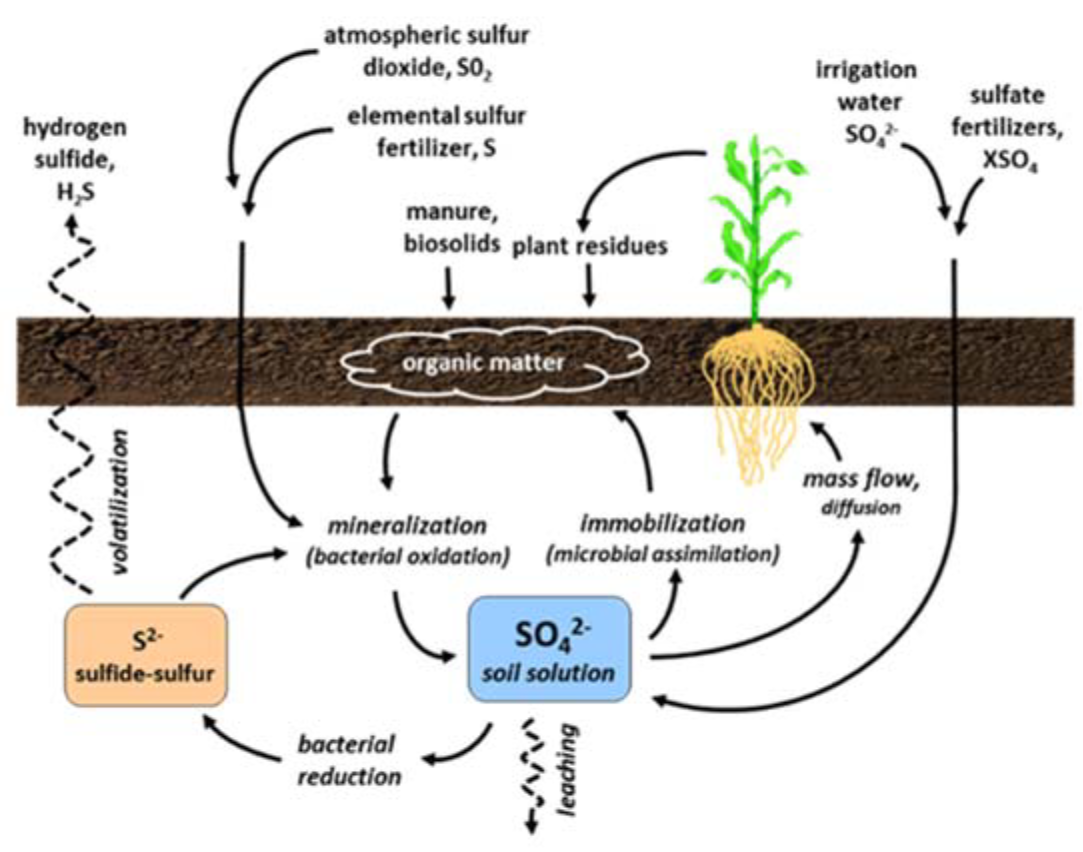
¶ Figure 1. Sulfur Sources
¶ B. Adsorption ↔ desorption
- Adsorbed SO42- ions are considered pool of “labile” sulfur
- Readily displaced from exchanged sites into soil solution, becoming plant available
- Adsorbed sulfate ions replenish sulfate ions removed from soil solution by plant uptake or other processes
- Most important in highly weathered soils
- Weathered soils have high adsorption potential
- Strength of adsorption
- SO42- > NO3-
- H2PO4- > SO42-
- Factors affecting adsorption capacity
- Soil pH
- Overall charge of soil colloid exchange surfaces is usually negative; provides cation exchange sites
- Sulfate adsorption capacity is greatest in acid soils
- At low pH, soil charge becomes more positive; provides anion exchange sites
- pH > 6.5, very low sulfate adsorption
- At high pH, negative charge increases, adsorption decreases
- Soil colloids[1]
- Hydrous oxides: iron and aluminum oxides are primary adsorption sites in many soils
- Soil clay content and clay mineralogy
- Absorption potential higher for 1:1 clay minerals (e.g., illite, kaolinite)
- Other anions and cations in solution affects adsorption
- Increased SO42- ion in solution increases adsorbed sulfate
- Sulfate ion considered weakly held
- Strength of adsorption: OH- > H2PO4- > SO42- > C3H3O2-> NO3- = Cl-
- Cations associated with sulfate anions affect amount of sulfate that is adsorbed
- Non-adsorbed sulfate ions remain in solution and can potentially be leached
- Strength of adsorption: H+ > Ca2+ > Mg2+ > K+ = NH4+ > Na+
- Soil pH
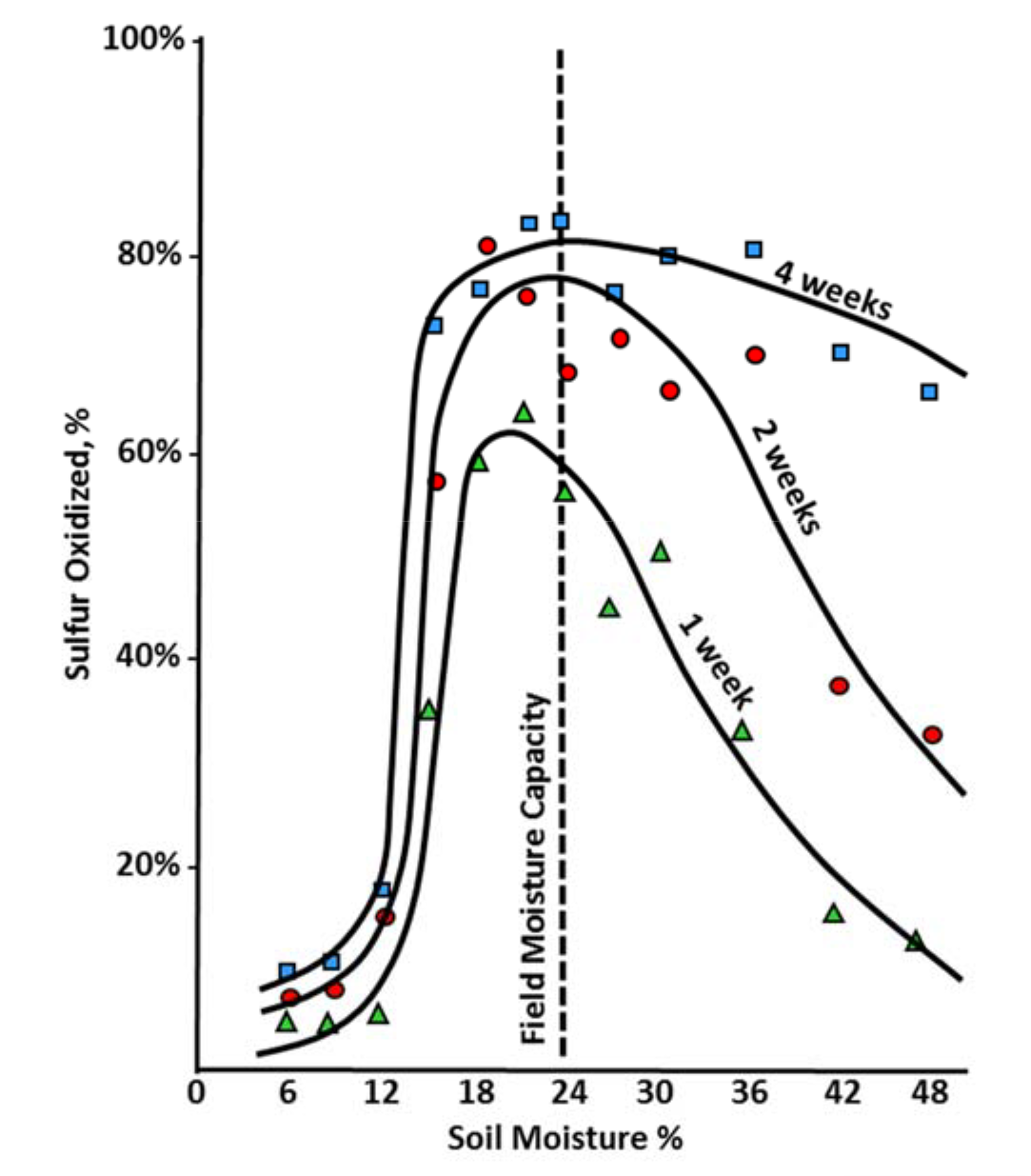
¶ Figure 2. Percentage of Added Elemental Sulfur Oxidized to Sulfate in a Miami Silt Loam
¶ C. Precipitation ↔ Dissolution
- Gypsum (CaSO4) is primary mineral in arid climates
- Co-precipitates (co-crystallizes) with calcium carbonates in calcareous soils
- Fine sulfate particles can result from grinding soil samples for testing
- Can overestimate plant-available sulfur
- Sulfides precipitate in anaerobic, waterlogged soils
- Hydrogen sulfide gas (H2S) precipitates as iron sulfide (FeS) and other metal sulfide minerals
- Dissolution of metal sulfides requires sulfur oxidation
¶ D. Oxidation ↔ Reduction
- Sulfur exists in a number of different oxidation states
- Sulfides, polysulfides, elemental sulfur, thiosulfate, sulfites, sulfates
- Valence ranges from -2 (sulfide) to +6 (sulfate)
- Sulfur oxidation and reduction are both affected by microbial processes
- Oxidation of elemental sulfur by sulfur oxidizers produces sulfate
- Reduction of sulfate by microbial processes produces sulfide
- Sulfur reduction
- Two definitions of sulfur reduction
- Microbial processes that convert sulfate to sulfide to help produce energy
- Set of forward and reverse pathways that progress from uptake and release of sulfate by cells to conversion of various sulfur intermediates and ultimately to sulfide which is released from cell
- Sulfate reducing bacteria “breathe” sulfate instead of oxygen
- Reduced inorganic sulfur compounds found in soil
- Occur in waterlogged, anaerobic conditions
- Wetlands, swamps, tidal marshes
- Two definitions of sulfur reduction
- Sulfur oxidation
- Sulfides and elemental sulfur are readily oxidized to sulfate in aerobic conditions
- Carried out by autotrophic and heterotrophic microbes
- Thiobacillus sp.
- Chemical oxidation occurs, but very slow process
- Sulfate (SO42-) is most oxidized form
- Plants must reduce SO42- ion to sulfhydryl groups (R-SH) for incorporation into most organic compounds (assimilation)
- Oxidation of hydrogen sulfide (H2S) produces elemental sulfur (S0)
- Reaction occurs in certain bacteria
- Elemental sulfur often stored as polysulfides
- Acidifying reactions
- Oxidation of elemental sulfur
- Used for acidification of agricultural or horticultural soils
- S2 + 3O2 + 2H2O → 2H2SO4 → 4H+ + 2SO42-
- Involves Thiobacillus sp. bacteria
- Affected by temperature, moisture, aeration
- Optimal soil moisture near field capacity (see Figure 2.)
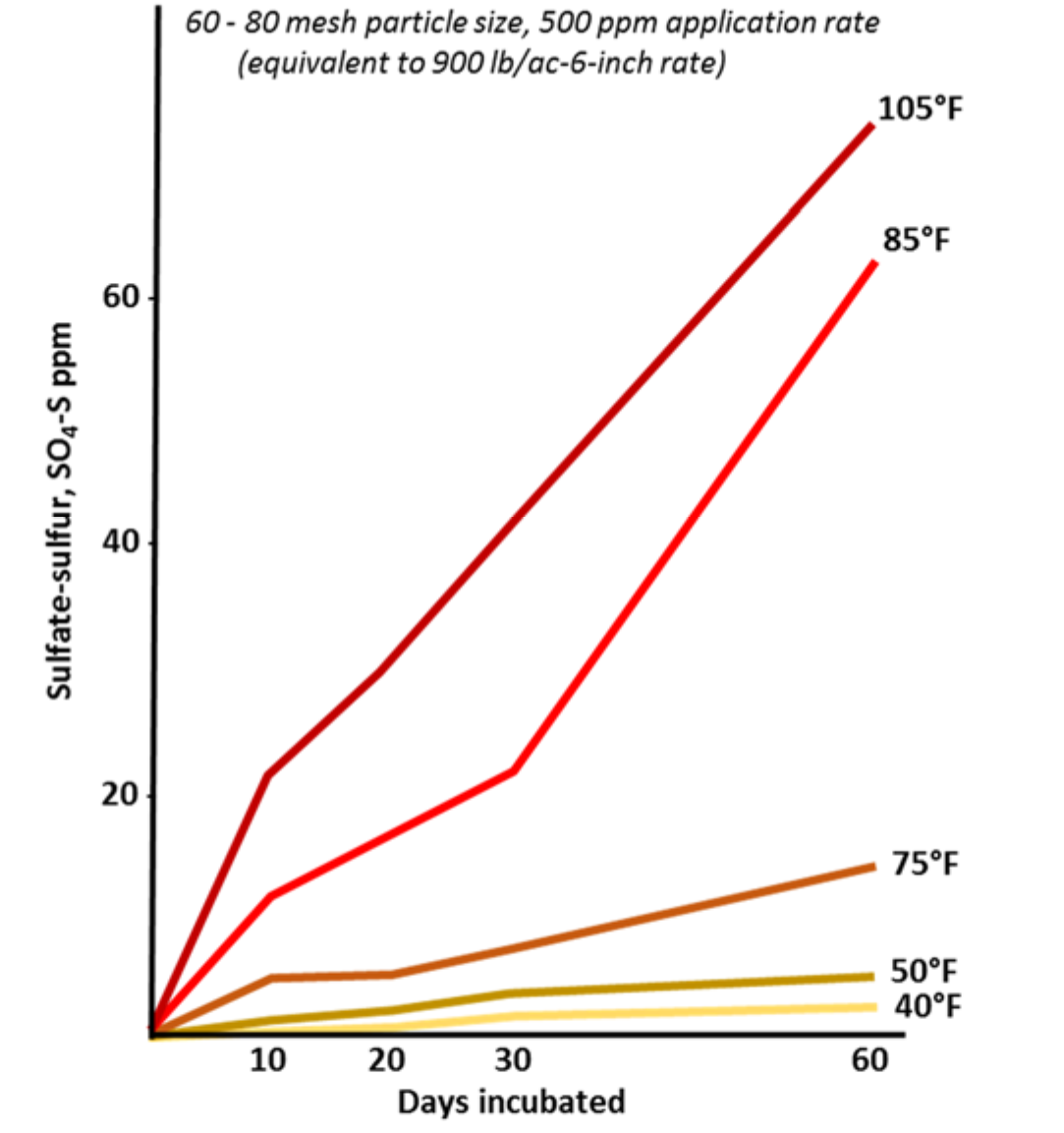
- Optimal temperature ranges from 75° to 105°F (25° to 40°C) (see Figure 3.)
- Oxidation of hydrogen sulfide
- H2S + 2O2 → H2SO4 → 2H+ + SO42-
- Oxidation of sulfides like pyrite (FeS)
- Common cause of acid mine drainage
- Can cause environmental problems
- Oxidation of elemental sulfur
¶ Figure 3. Elemental Sulfur Oxidation as Affected by Temperature
¶ E. Volatilization
- Volatile sulfur losses
- Impact on soil fertility generally insignificant
- Products of microbial transformations in soil
- Major product is dimethyl sulfide (CH3SCH3)
- Also carbon disulfide, methyl mercaptan, and dimethyl disulfide
- Elemental sulfur can be reduced to hydrogen sulfide
- Desulfurization
- Organic molecules containing sulfur desulfurized to hydrogen sulfide gas (H2S)
- Sulfate-reducing microbes can generate hydrogen sulfide from sulfate
- Volatile sulfur additions
- Atmospheric SO2
- Result of burning fossil fuels, other industrial activities
- Deposited through rainfall
- Contributes to acid rain
- Significant source of sulfur fertility in some areas
- Contribution to sulfur declining due to low-sulfur air emissions regulations
- Atmospheric SO2
- Direct volatilization from plant leaves also occurs
- Can affect pasture and hay palatability
[1] Colloid = Mixture in which one substance of microscopically dispersed insoluble particles is suspended throughout another substance; particles do not settle and cannot be separated out. Example, milk is an emulsified colloid of liquid butterfat globules dispersed within a water-based solution.