⇦ Back to Soil Fertility and Plant Nutrition Home
¶ Sulfur in the Plant
- Required by plants in relatively large amounts
- Less sulfur required than nitrogen or potassium
- Amounts required are similar to phosphorus, calcium, or magnesium (See Crop File 1.02.034, Nutrient removal values: Sulfur, Zinc)
- Component of various plant proteins
- About 90% of plant sulfur content is in protein forms
- Essential amino acids
- Cystine, cysteine and methionine
- Disulfide (- S - S -) linkages
- Protein configuration and enzyme activity
- Synthesis of chlorophyll
- Ferredoxin
- Fe-S protein
- Redox reactions
- Photosynthesis, nitrogen fixation
- Nitrate and sulfate reduction
- Coenzymes
- Coenzyme A
- Vitamins: biotin, thiamine, B1
- Volatile compounds
- Present in onions and in crucifer family plants
- Plant uptake
- Most sulfur taken up by plant roots as inorganic sulfate anion, (SO42-)
- Less than 10% of total soil sulfur is in sulfate form
- Soils in arid climates often have elevated sulfate content
- Sulfur dioxide (SO2) can be absorbed directly by leaves
- Typically absorbed in small amounts
- High concentrations are toxic
- Relatively immobile in plant
- Not readily translocated from older leaves to young growing points
- Most sulfur taken up by plant roots as inorganic sulfate anion, (SO42-)
- 7. Deficiency symptoms
- Usually observed first in newer growth
- Stunted, spindly growth
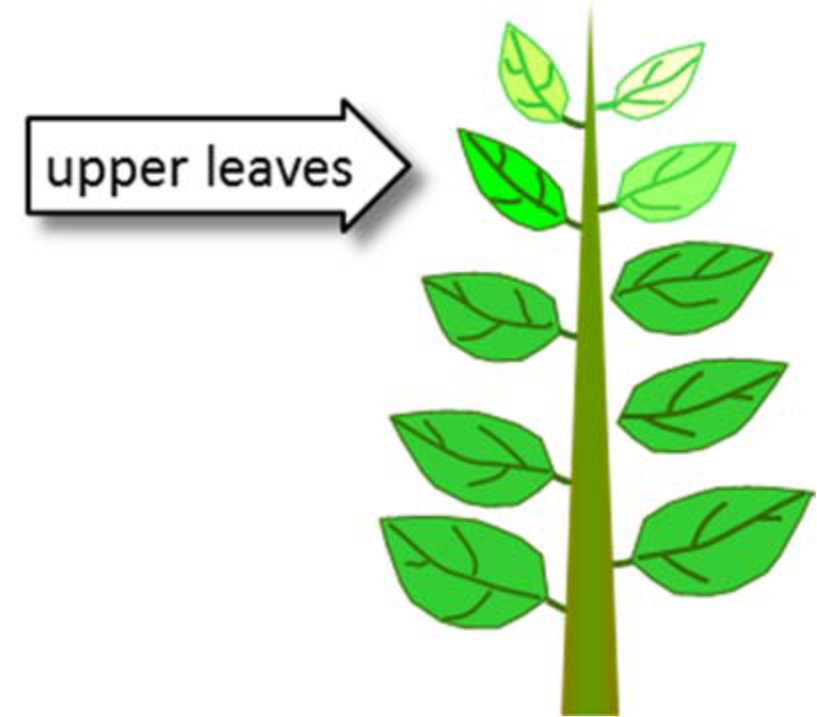
- Yellowing, chlorosis of upper, younger leaves
- Can be confused with nitrogen deficiency
- Sulfur deficiency – upper leaves
- Nitrogen deficiency – lower leaves
- Often entire plant is uniformly chlorotic
- Usually occurs early in growing season
- May occur simultaneously with nitrogen deficiency
- Low protein; accumulation of non-protein nitrogen
- Crucifers develop a reddish, purple discoloration
- Usually observed first in newer growth
¶ Figure 1. Sulfur Deficiencies
¶ B. Sulfur in the Soil
- Sulfate ion (SO42-) moves by both mass flow and diffusion
- Primarily moves to plant roots by mass flow
- Diffusion important in low sulfur soils
- Soil solution concentrations between 5 and 20 ppm SO4-S are common in temperate region agricultural soils
- Crop requirements generally met by 3 to 5 ppm SO4-S in soil solution
- Concentration may be less than 5 ppm SO4-S in sandy, low sulfur soils
- Primarily moves to plant roots by mass flow
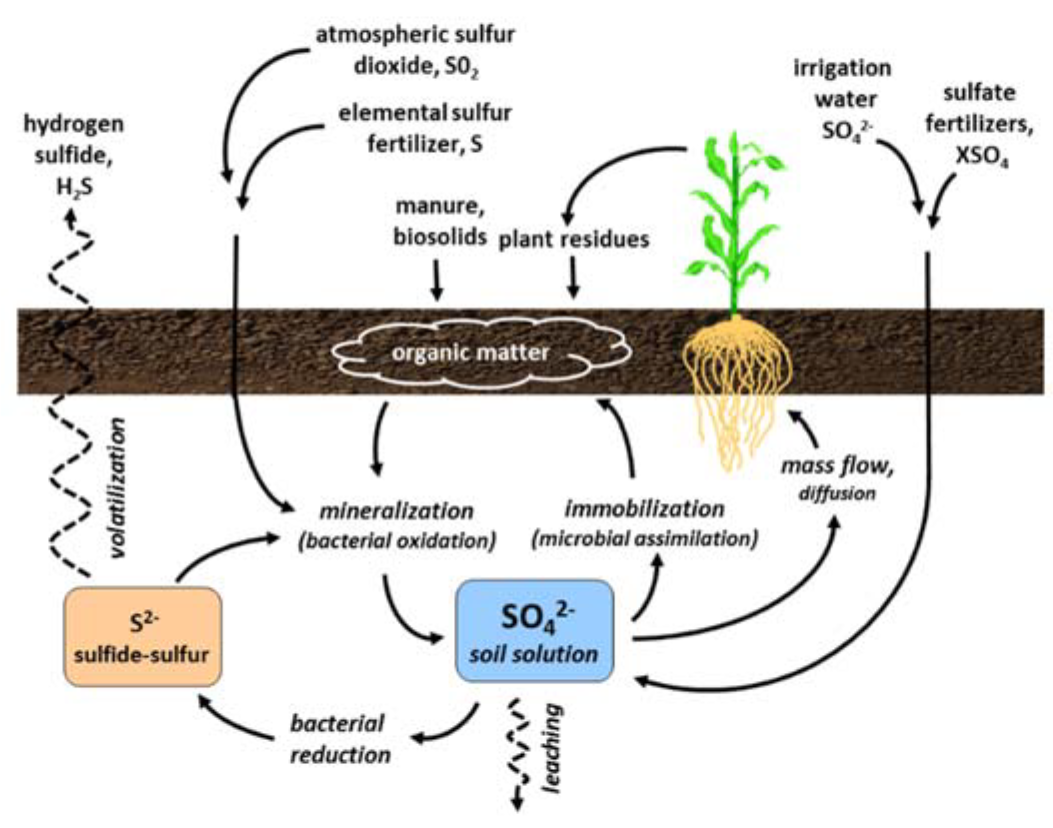
- Sources in the soil
- About 90% of soil sulfur is in organic form
- Soil organic matter about 1% sulfur in different forms
- Sulfate esters and ethers
- C - O - S linkages
- About 30% to 60% of organic sulfur content
- Amino acids
- Also includes other C- S compounds
- About 10% to 20% of organic sulfur content
- Residual sulfur
- Are unidentified compounds
- About 30 to 40% of organic sulfur content
- Sulfate esters and ethers
- Adsorbed sulfate, SO42-
- Sulfate anion can be adsorbed and desorbed from exchange sites in extremely acidic soils
- Anion exchange capacity increases with decreasing pH
- Frequently adsorbed to iron and aluminum oxide mineral surfaces
- Sulfate anion can be adsorbed and desorbed from exchange sites in extremely acidic soils
- Sulfur minerals
- Gypsum (calcium sulfate) accumulations frequently found in soil of dry climates
- Accumulations found in both surface soil and subsoil
- Sulfides found under anaerobic conditions
- Gypsum (calcium sulfate) accumulations frequently found in soil of dry climates
- Sulfur transport
- Erosion
- Loss of organic matter depletes soil sulfur
- Leaching
- SO42- ion is highly mobile
- Often a major anion in water percolating through the soil and moving through drainage systems
- Losses affected by solution cations
- Leaching potential is highest when monovalent cations (K+, Na+) are high
- Volatilization losses
- As hydrogen sulfide (H2S) and other gases
- Erosion
¶ Figure 2. Sources of Sulfur
¶ C. Sulfur Transformations in Soil
(More details provided in Crop File 1.04.551, Sulfur Transformations in the Soil)
- Mineralization ↔ immobilization
- Mineralization: Release of organic sulfur as inorganic, plant-available SO4-S
- Immobilization: Uptake of inorganic sulfur from the soil and assimilation into organic sulfur forms by microbial activity
- Adsorption ↔ desorption from exchange surfaces
- Affected by:
- Soil colloids
- Soil pH
- Soil solution composition
- Affected by:
- Precipitation ↔ dissolution of secondary minerals
- Gypsum (CaSO4) in arid climates
- Sulfides in anaerobic, waterlogged soils
- Oxidation ↔ reduction reactions
- Sulfur exists in a number of different oxidation states
- Sulfides, polysulfides, elemental sulfur, thiosulfate, sulfites, sulfates
- Affected by:
- Microbial processes
- Soil water content
- Soil aeration
- Sulfur exists in a number of different oxidation states
- Volatilization
- Volatile sulfur losses
- During microbial transformations
- Direct volatilization from plant leaves
- Volatile sulfur additions
- Atmospheric sulfur dioxide (SO2) deposited through rainfall
- Volatile sulfur losses
¶ D. Sulfur Deficiency Conditions
- Sandy soils most often sulfur deficient
- Low organic matter, typically less than 2%
- High leaching potential due to coarse texture
- Deficiencies often observed in patches within field
- Weather conditions affect deficiency
- Mineralization from organic matter delayed in cool or cold soils
- High leaching potential due to excess precipitation
- Delayed root growth slows contact with and utilization of subsoil sulfate
- Crop sulfur requirements vary widely
- Requirement comparatively high for alfalfa, clovers, canola, cabbage and related vegetables, forage Brassica species, onions, garlic
- Corn, canola, winter cereals, and spring grains can be sensitive to deficiency conditions
- More pronounced with cool soils, excess moisture, and/or sandy soils
- Grass/legume pasture/hay
- Grasses absorb sulfur faster than legumes
- Cool season grasses more prone to deficiency than warm season grasses
- Legumes do not persist in grass/legume mixture if soil sulfur is low
- Grasses absorb sulfur faster than legumes
- Reduced deposition through rainfall
- Sulfur emissions from burning coal were significant source of sulfur fertility in some areas
- Contributed to acid rain
- Air quality regulations resulted in reduced emissions
- Corresponding reduction in sulfur deposition
- ii. Deficiencies reported more frequently
- Sulfur emissions from burning coal were significant source of sulfur fertility in some areas
- Historical transition to fertilizers with lower sulfur content
- Single superphosphate (16-20-0), 12% to 14% S
- Triple superphosphate (0-44-0), 0% to 3% S
- Diammonium phosphate (18-46-0), 0% S
- Ammonium polyphosphate (10-34-0), 0% S
¶ E. Sulfur Excess Conditions
- Not directly toxic to plants or other organisms
- Sulfate can contribute to soluble salt problems
- Is major anion in saline soils
- Significant ion in some irrigation waters
- High sulfate leaching conditions can also increase cation losses
- Sulfur environmental issues are not due to agriculture
- Acid mine drainage is primary concern
¶ F. Soil Testing for Sulfur
- Sulfur soil tests typically not well correlated with crop response
- Other factors essential for interpretation
- Soil texture: sandy vs. loamy or clayey
- Organic matter: responses when less than 2% OM
- Responsiveness of crop species
- Weather conditions before and during growing season
- Subsoil sulfate concentration
- Monocalcium phosphate extraction
- Measures readily soluble and adsorbed sulfate
- Determined by turbidity or by ion chromatography
- Ammonium acetate extraction
- Same extraction used for potassium and other cations
- Determined by inductively coupled plasma (ICP)
- Correlated to calcium phosphate method, but results are slightly higher
- Measures sulfate and possibly some forms of readily mineralizable sulfur
- Mehlich-3 extraction
- Well correlated to ammonium acetate method
- Results similar to ammonium acetate results
- Other factors essential for interpretation
¶ G. Sulfur Nutrient Sources
- Organic, biological sources
- Manure, compost, biosolids
- 1 to 5 lb S per wet ton
- Soil organic matter
- Estimated mineralization: About 3 lb S per each 1% OM per acre per year
- Manure, compost, biosolids
- Inorganic (non-fertilizer) sources
- Atmospheric deposition:
- Less than 2 to 4 lb S/ac/yr
- Irrigation water
- Each 1 mg/L SO4-S (3 mg/L SO4) in water equivalent to 0.23 lb S per acre-inch applied as irrigation
- If irrigation water analysis more than 6 to 8 ppm SO4-S (18 to 24 ppm SO4), reduce recommended fertilizer sulfur by half
- Atmospheric deposition:
- Ammonium sulfate (AMS)
- Prills or water-soluble crystals, (NH4)2SO4
- Common analysis: 24% S, 21% N
- Also used as additive in herbicide spray solutions
- Ammonium thiosulfate (ATS)
- Clear liquid product, (NH4)2S2O3
- Common analysis: 26% S, 12% N
- Commonly used to add sulfur to starter fertilizers
- Often mixed with UAN solution for fertigation
- 80% UAN + 20% ATS ≈ 20-0-0-5S fertilizer solution
- Not recommended for direct seed placement
- Potentially damaging to seedling root and shoot tissues
- Potassium thiosulfate (KTS)
- Clear liquid product, K2S2O3
- Common analysis: 17% S, 25% K2O
- Major use: adding potash and sulfur to UAN solution (e.g., 28-0-0) and to starter fertilizer materials
- Use caution when blending with UAN solution; may form KNO3 crystals
- Not recommended for direct seed placement
- Potentially damaging to seedling root and shoot tissues
- Potassium-magnesium sulfate
- Granular product, 2MgSO4•K2SO4
- Common analysis: 22% S, 22% K2O, 18% MgO
- Totally soluble, but slower to dissolve than other sources
- Common trade names: langbienite, sulfate of potash-magnesia, K-Mag, Sul-Po-Mag
- Magnesium sulfate
- Epsom salts, common mineral, MgSO4•7H2O,
- Common analysis: 13% S, 10% Mg
- Highly soluble
- Magnesium sulfate, monohydrate
- Kieserite, MgSO4•H2O
- Common anlysis: 23% S, 17% Mg
- Epsom salts, common mineral, MgSO4•7H2O,
- Potassium sulfate (SOP)
- Water soluble granular product, K2SO4
- Common analysis: 17% to 18% S, 48% to 53% K2O
- Common trade name: sulfate of potash (SOP)
- “Salt” measurement (EC) result from K2SO4 solution less than one-third of KCl solution
¶ H. Sulfur-Containing Soil Amendments
- Primary uses are for soil improvement
- To acidify (reduce pH) of neutral to calcareous soils
- Extract soluble calcium from soil carbonates (excess lime) to replace sodium ions on soil clay exchange surfaces
- Providing sulfur as nutrient often is secondary benefit
- Gypsum, CaSO4•2H2O
- Common analysis: 18% S, 22% Ca
- Used to remediate sodium-affected soils (sodic soils)
- Economical source of soluble calcium for sodic soil remediation
- CaSO4 + 2Na-clay → Ca-clay + Na2SO4
- Does not directly affect soil pH
- Removing sodium may mitigate pH of sodic soil by suppressing sodium bicarbonate formation
- Gypsum does not change pH of “normal” or non-sodic soil
- Available in granular, fine crystals, or pelletized forms
- Often broadcast applied to soil surface
- Can be slurried and injected into irrigation water
- Elemental sulfur, S
- Several physical forms
- “Flowers of sulfur”
- Fine powder, 100% S
- “Dusting” sulfur used as fungicide
- Prilled sulfur sources
- Typically 90% sulfur, 10% bentonite
- Sized for blending with other prilled fertilizer materials
- Imbibes moisture, prill disintegrates, fine sulfur particles remain
- Flowable sulfur (52% to 70% S) used as foliar fungicide
- Requires adequate agitation
- Can be applied with sprinkler irrigation
- “Flowers of sulfur”
- Elemental sulfur not immediately plant-available
- Must be oxidized from elemental sulfur to sulfate form
- Particle size important; dust-sized particles react most rapidly
- Initial soil reaction is acidification
- 2S0 + 3O2 + 2H2O → [Thiobacillus species] → 2H2SO4 → 4H+ + 2SO42-
- Subsequent reaction in calcareous soil dissolves carbonates; results in gypsum
- i. 2H+ + SO42- + CaCO3 → CaSO4 + CO2 + H2O
- Several physical forms
- Urea-sulfuric acid (USA)
- Liquid materials, (NH2)2CO•H2SO4
- Common analysis: 10-0-0-18S, 15-0-0-16S, 28-0-0-9S
- Considered an “adduct”
- Typically added to irrigation water
- Reacts with bicarbonate, HCO3-
- Removes scale, cleans irrigation lines
- May be corrosive to metals, depending on specific formulation
- Reaction in calcareous soils
- (NH2)2CO•H2SO4 + CaCO3 + 2NaX → CaX2 + (NH2)2CO + Na2SO4 + CO2 + H2O
- Can provide soluble calcium for sodic soil remediation
- Removing sodium can mitigate pH of sodic soil
- Ammonium polysulfide (APS)
- Liquid solution: 40% S, 20% N
- Polysulfide must be oxidized to sulfate for root uptake
- Reaction in calcareous soils
- (NH4)2S5 + 8O2 + 4CaCO3 + 8NaX → 4CaX2 + (NH4)2SO4 + 4CO2
- Provides soluble calcium for sodic soil remediation
- Often added to irrigation water
¶ I. Sulfur nutrient management
- Wide variety of sulfur fertilizer sources
- Sulfate sources equivalent in availability
- Other sources must be converted to sulfate
- Timing
- Mobile nutrient; must be applied annually, like nitrogen
- Deficiencies occur early in growing season
- Annual row crops: Apply shortly before planting, at planting, during early and mid-vegetative stages
- Small grains and cereals: Apply before dormancy breaks
- Perennial forage crops: Apply in early spring before annual growth begins
- Fertigation
- Apply before or in early portion of rapid vegetative growth stages
- e.g., 6-leaf to 8-leaf corn
- Follow-up application may be necessary with sandy, low organic matter soils
- Apply before or in early portion of rapid vegetative growth stages
- Elemental sulfur or sulfide materials require time for conversion to sulfate
- Must be soil incorporated
- Soil moisture, adequate aeration necessary for microbial activity
- May require 2 to 6 months for complete reaction
- Reaction is faster in warmer soil conditions; faster in summer than winter
- Must be soil incorporated
- Placement
- Surface broadcast and subsurface banded applications of sulfate-type fertilizers appear equally effective
- Sulfur as sulfate is mobile nutrient
- Can be leached below root zone under right conditions
- Elemental or sulfide-type fertilizers require some method of soil incorporation
- Must be converted to sulfate form
- Caution required with direct seed placement
- Sulfate fertilizers or sulfate-generating fertilizers may contribute to “salt” injury
- Avoid thiosulfate fertilizer sources
- Can be toxic and potentially damaging to seedling root and shoot tissues
- Surface broadcast and subsurface banded applications of sulfate-type fertilizers appear equally effective