⇦ Back to Soil Fertility and Plant Nutrition Home
¶ Magnesium in the Plant
- Considered an essential secondary macronutrient
- Required by plants in relatively large amounts
- Typical concentration in plants 0.15% to 0.40% Mg
- Less magnesium required than nitrogen or potassium
- Plant requirements similar to phosphorus, sulfur, and calcium, but usually less magnesium required than calcium
- Essential for photosynthesis; responsible for electron transfer
- Central element of chlorophyll molecule
- Chlorophyll contains 10% to 25% of total plant magnesium
- Required for protein synthesis; structural component of ribosomes
- Enzyme activation
- Catalyst for phosphate transfer reactions involving energy synthesis (ATP ↔ ADP)
- Essential for carbon dioxide fixation; activates carboxylase enzymes
- Mobility within the plant
- Taken up by roots as a divalent cation, Mg2+
- Mobile in plant
- Translocated from older leaves to young growing points
- Deficiency symptoms
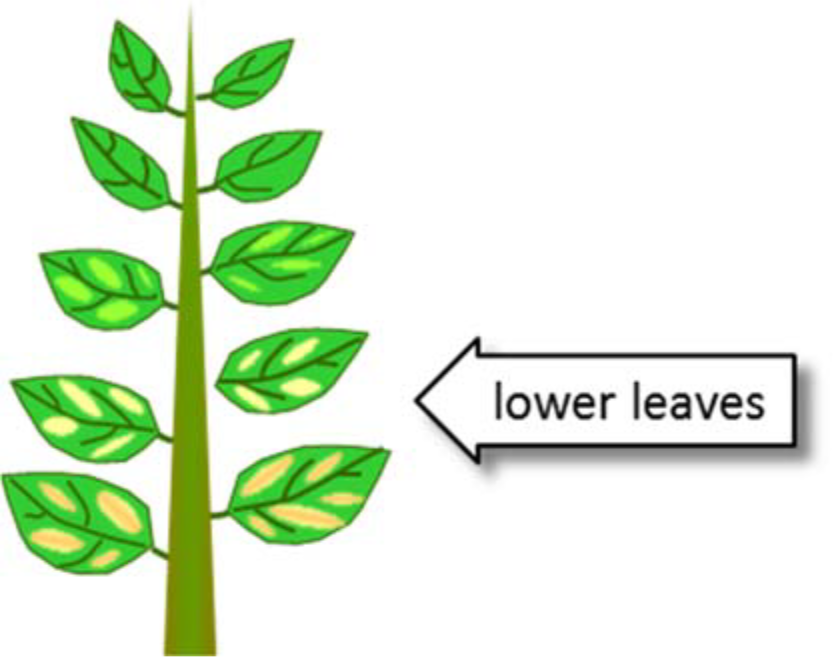
- Occurs first on older, lower leaves
- Interveinal chlorosis (yellowing) is primary symptom of many plants
- May be confused with iron deficiencies
- Interveinal yellowing progresses from leaf edge to center
- Lower leaves on other species may develop reddish-purplish cast with necrotic margins
- Leaf necrosis in advanced stages
- Leaves may become stiff and brittle; veins twisted
- Excess magnesium
- Not directly toxic to plants or other organisms
- Excess magnesium is stored in plant vacuoles
- High soil magnesium can inhibit uptake of other cations
- May induce potassium deficiency or calcium deficiency in some situations
- Not directly toxic to plants or other organisms
¶ Figure 1. Magnesium Deficiencies
¶ B. Magnesium in the Soil
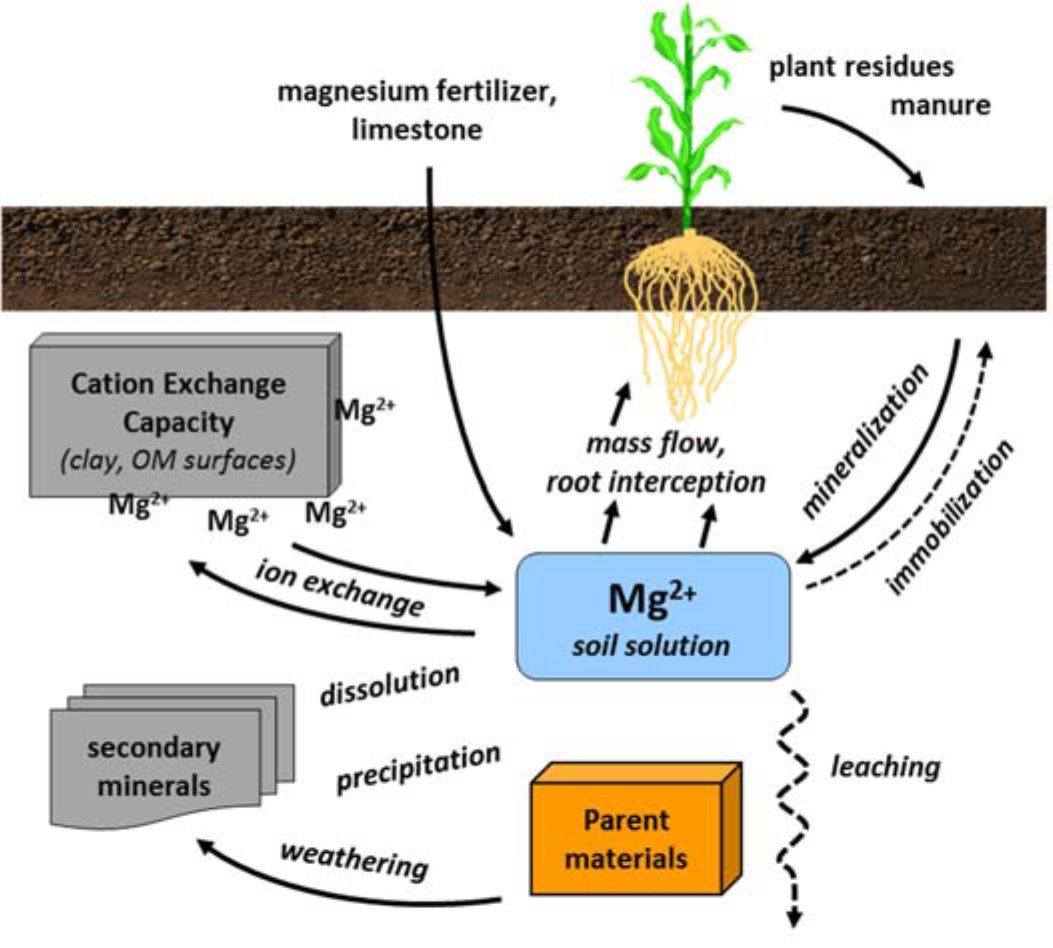
- Mg2+ is an exchangeable cation
- Cation exchange is most important magnesium reaction in soil
- Passive uptake of Mg2+; supplied primarily by mass flow, but also root interception
- More magnesium moves to roots by mass flow than is taken up
- Root interception much lower than for calcium
- Uptake declines quickly below pH 5.5
- Soil solution concentrations often between 5 and 50 ppm Mg in temperate region soils
- High levels of other cations in soil solution may inhibit Mg2+ uptake
- Other cations are primarily Ca2+, K+, NH4+, Al3+
- Derived from weathering and dissolution of magnesium minerals
- Constituent of primary minerals; e.g., dolomite, biotite, hornblende, olivine, serpentine
- Constituent of secondary clay minerals; e.g., illite, chlorite, vermiculite, montmorillonite
- Availability affected by precipitation ↔ dissolution of lime and secondary minerals
- Dolomitic limestone
- Clay minerals high in magnesium; e.g., 2:1 clays like vermiculite
- Lower magnesium concentrations more common in coarse-textured than in fine-textured soils
- Soils of arid or semi-arid regions often have high magnesium levels
- Organic matter
- Much of the magnesium is readily leached from crop residues
- Most of the remainder is mineralized during early stages of residue decomposition
- Cation exchange
- Cation exchange reactions dominate magnesium behavior in soil
- Adsorption ↔ desorption from clay and organic matter surfaces
- About 5% of soil magnesium is exchangeable Mg2+
- Is rapid equilibration between exchangeable magnesium and solution magnesium
- Mg2+ held more strongly than monovalent cations
- Al3+ > Ca2+ > Mg2+ > K+ = NH4+ > Na+
- Exchangeable Mg2+ buffers the solution Mg2+ pool
- Depends on quantity and intensity factors
- Cation exchange reactions dominate magnesium behavior in soil
¶ Figure 2. Magnesium Sources
¶ C. Magnesium Deficiency Conditions
- Acid, sandy, low CEC soils with high leaching most prone to deficiency
- Less available at pH below 5.5
- Aluminum ion (Al3+) present in soil solution under low pH, acidic soil conditions inhibits Mg2+ ion uptake
- Leaching
- Magnesium often a major cation in water percolating through the soil and moving through drainage systems
- Long-term loss of Mg2+ ion by leaching contributes to soil acidification
- Potassium fertilizers (e.g., KCl and K2SO4)
- Can increase potential losses in low magnesium soils by displacing exchangeable magnesium; more prone to leaching
- High soil potassium can also induce magnesium deficiency by inhibiting uptake
- Erosion losses of magnesium in sediment from high CEC soils can be large
¶ D. Soil Testing for Magnesium
- Usually expressed as “exchangeable Mg” concentrations
- Includes both water soluble and exchangeable forms
- Mg2+ ions on exchange sites displaced by cation in extracting solution
- Common critical range from 20 to 50 ppm Mg
- Analytical methods
- Ammonium acetate (1N, pH 7.0) extraction
- Uses NH4+ as extracting cation to displace Mg2+
- May include small amounts of magnesium dissolved from carbonates in calcareous soils
- Mehlich-3 (pH 2.5) extraction
- Uses NH4+ as extracting cation to displace Mg2+
- Frequently includes magnesium dissolved from carbonates in calcareous soils
- Other methods may use NH4+ or Na+ as extracting cation
- Concentrations in filtered extract determined by atomic absorption spectroscopy (AAS) or inductively coupled plasma spectrometry (ICP)
- Ammonium acetate (1N, pH 7.0) extraction
- Magnesium saturation
- Magnesium considered “basic” cation
- Exchangeable Mg concentration used in calculations for cation exchange capacity (CEC) and base saturation
- % Mg saturation = [(Mg ppm / 120) / CEC as mEq per 100g] * 100
- Wide range of calcium-to-magnesium ratios have no effect on plant growth as long as soil magnesium levels not deficient (e.g., over 50 to 100 ppm exchangeable Mg)
- Sodic (sodium-affected) soils
- Excess sodium ions in soil solution may be more competitive against magnesium ions adsorbed to exchange sites than to calcium ions
- Irrigating with high magnesium water (Ca:Mg ratio greater than ”2”) could slightly aggravate sodium symptoms
¶ E. Magnesium Nutrient Management
- Organic, biological sources
- Manure, compost, biosolids
- Manure may supply 2 to 10 lb Mg per wet ton
- Most magnesium is in soluble forms, readily available
- Much of magnesium is readily leached from crop residues
- Manure, compost, biosolids
- Inorganic nutrient sources
- Dolomitic limestone, MgCO3•CaCO3
- Common analysis: 6% to 20% Mg, 24% to 30% Ca
- Langbienite, 2MgSO4•K2SO4
- Common analysis: 11% Mg, 22% S, 20% to 22% K2O
- Trade names include Sul-Po-Mag®, K-Mag®
- Totally soluble, but slower to dissolve than other sources
- Magnesium sulfate, MgSO4•7H2O,
- Common analysis: 10% Mg, 13% S
- Epsom salts, common mineral
- Highly soluble
- Magnesium chloride, MgCl
- Common analysis: 25% Mg
- Highly soluble, frequently used in liquid fertilizers
- Kieserite, MgSO4•H2O,
- Common analysis: 17% Mg, 23% S
- Magnesium sulfate, monohydrate
- Kainite, MgSO4•KCl•3H2O
- Common analysis: 9% Mg, 18% K2O
- Mixed salt, variable solubility
- Magnesium oxide, MgO
- Common analysis: 56% Mg
- Formed by heating MgCO3 to drive off carbon dioxide
- Rather insoluble
- Struvite, MgNH4PO4•6H2O
- Common analysis: 10% Mg, 5% N, 27% P2O5
- ii. Produced primarily during phosphorus recovery in livestock or municipal wastewater sources
- iii. Dissolves slowly
- Irrigation water
- Each 1 mg/L Mg in water equivalent to 0.23 lb Mg per acre-inch applied as irrigation
- Dolomitic limestone, MgCO3•CaCO3
- Placement
- Surface broadcast applications must be soil incorporated
- Magnesium considered immobile nutrient
- Make soil applications before planting
- Surface broadcast and banded applications considered equally effective
- Including low rate in starter fertilizer plus broadcast application may be beneficial in deficient soils
- Surface broadcast applications must be soil incorporated
- Rates
- Soil application rates based on “build” approach
- One-time application to increase existing soil test level past critical level into optimum level
- Recommended application usually considered adequate for following three to five years
- Magnesium applied to soil is frequently included in materials used to neutralize acidity
- Magnesium easily managed with liming on low pH soils by using dolomitic limestone
- Liming has potential to induce magnesium deficiency, if high-calcium lime (calcite) is used on very low magnesium soils
- Soil application rates based on “build” approach
- Foliar application
- Complexing agents or chelates added to inorganic sources to improve foliar uptake
- May be used to temporarily correct deficiency, but must be repeated until soil has adequate magnesium
¶ F. Grass Tetany
- Livestock magnesium deficiency; low blood magnesium
- Possible concern with beef and dairy cattle, horses
- Affected by livestock age, lactation status, or breed.
- Most frequent when forage is lush and growing rapidly
- Can occur at magnesium levels in forage and pasture grasses that are not deficient
- More of a problem in pure grass pasture than grass-legume pasture.
- More economical to add magnesium salt to diet than magnesium fertilizer to soil
- Maintain proper nitrogen and phosphorus fertilizer rates for grazed forages
- Maintaining proper soil pH also important