⇦ Back to Soil Fertility and Plant Nutrition Home
¶ A. Calcium in the Plant
- Considered an essential secondary macronutrient
- Required by plants in relatively large amounts
- Less required than nitrogen or potassium
- Requirement similar to (or higher than) phosphorus, sulfur, and magnesium
- Plants usually contain 0.1% to 5.0% Ca in fresh weight
- Adequate levels in mature leaves typically ranges between 0.5% and 1.5% Ca
- Required by plants in relatively large amounts
- Forms and functions
- Most calcium is in cell walls and cell membranes
- Is an "apoplastic" nutrient
- Concentrations in cytoplasm (or “interior cell contents”) are low
- Mostly found outside of plasma membrane in cell walls
- Has limited role in metabolism
- Is an "apoplastic" nutrient
- Structural component of cell membranes and cell walls
- Calcium pectates help cement adjacent cell walls
- Improves strength of cell walls
- Provides resistance to fungal penetration and infection
- Provides membrane function and stability; helps maintain cell integrity
- Regulates selectivity of ion uptake
- Controls membrane permeability and prevents leakage of solutes
- Essential for cell elongation in shoot and in growing tips of roots
- Regulates growth and provides structure
- Calcium pectates help cement adjacent cell walls
- Most calcium is in cell walls and cell membranes
- Mobility
- Taken up by roots as a divalent cation, Ca2+
- Calcium supply via xylem (in transpiration stream) very important
- Xylem transport to leaves is “one-way ticket”
- Very immobile in plant
- Limited translocation by phloem from older leaves to developing tissues
- Can lead to inadequate calcium in fruits, tubers, and growing points of shoots and roots
- Deficiency can occur on high calcium soils, especially in parts of plant with low transpiration rates
- Taken up by roots as a divalent cation, Ca2+
- Deficiency symptoms
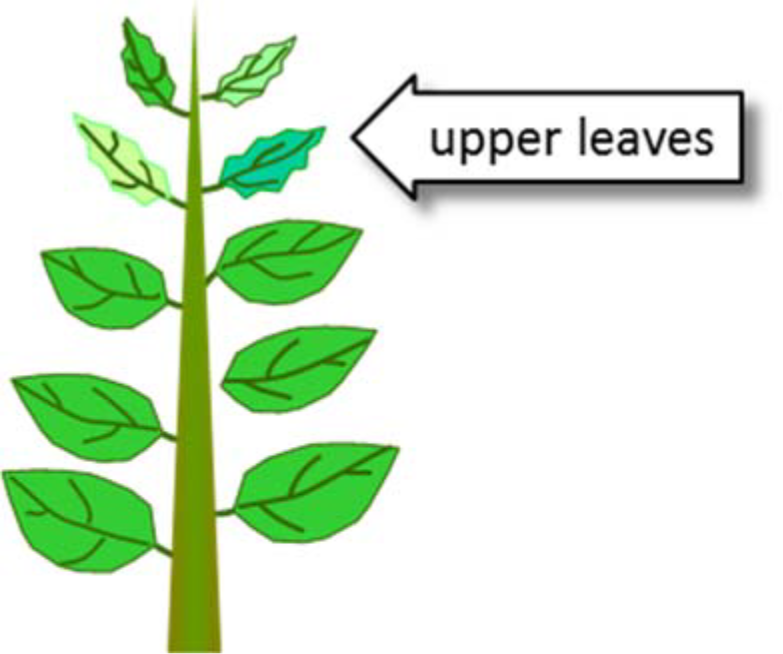
- Occurs first in growing root and shoot tips
- Occurs first in youngest leaves
- Leaves become deformed and chlorotic
- New leaves may not unfold
- Called “sticky leaf” or “buggy whipping” in corn
- Leaves may become necrotic in later stages
- Marginal burning, cupping of younger leaves
- Example: tip burn of lettuce
- Leaves become deformed and chlorotic
- Physiological disorders of storage organs
- Blossom end rot of tomato, pepper
- Bitter pit of apple
- Unfilled pods (“pops”); darkened plumules in the seed of peanuts
- Excess calcium
- Not directly toxic to plants or other organisms
- High soil calcium can inhibit uptake of other cations
- May induce potassium or magnesium deficiency
¶ Figure 1. Calcium Deficiencies
¶ B. Calcium in the soil
- Total calcium content depends on parent material and historical rainfall
- Tropical climate soils: 0.1% to 0.3% Ca
- Temperate climate soils: 0.7% to 1.5% Ca
- Calcareous soil content may be 1% Ca or more (over 2% to 3% CaCO3 equivalent)
- Taken up by roots as a divalent cation, Ca2+
- Ca2+ supplied by both root interception and mass flow
- Ca2+ accumulates around roots in soils with abundant calcium
- Ca2+ absorption is restricted to root tips
- Region of young roots where cell walls of endodermis are not yet suberized
- Suberization = conversion of cell walls into corky tissue by infiltration with suberin
- Ca2+ absorption is restricted to root tips
- Continuous root growth required for adequate calcium uptake
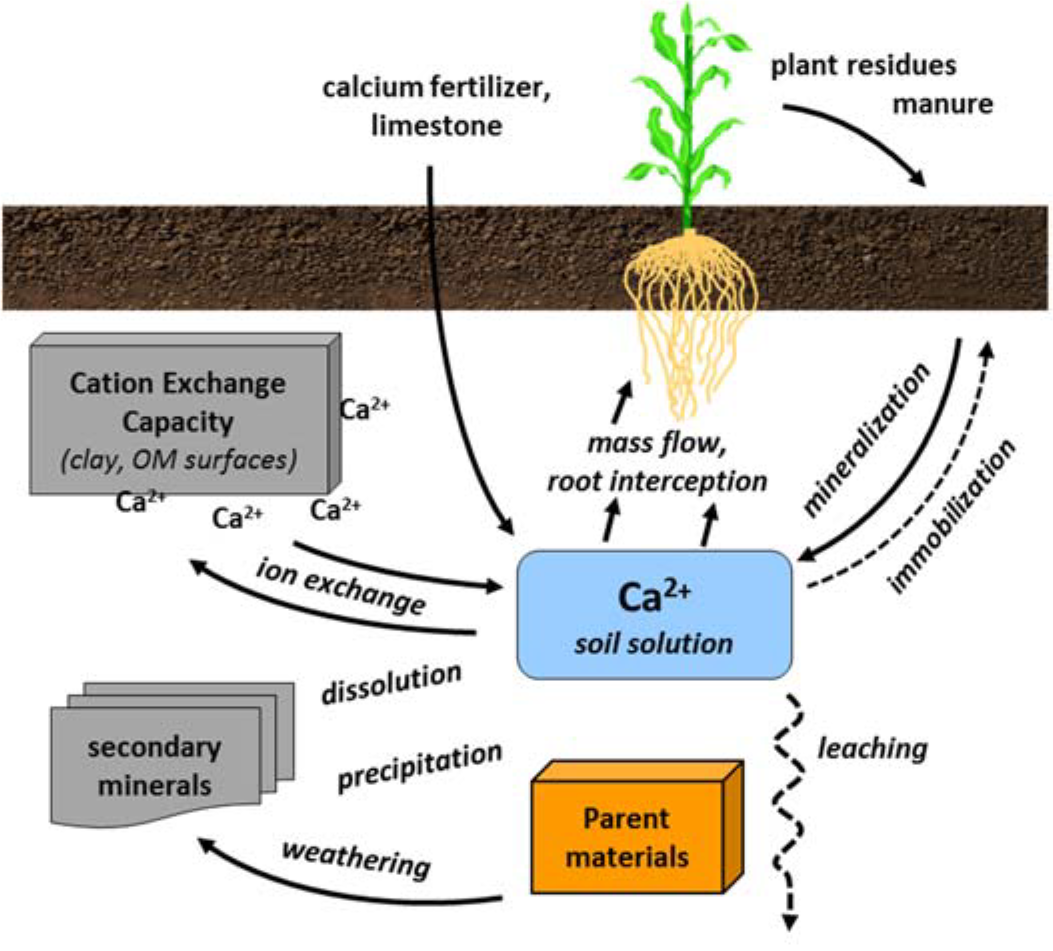
- Soil transformations
- Weathering ↔ formation of primary soil minerals
- Precipitation ↔ dissolution of lime and secondary minerals
- Carbonates
- Calcium phosphates
- Cation exchange
- Adsorption ↔ desorption from clay and organic matter surfaces
- Ca2+ is exchangeable cation
- Cation exchange reactions dominate calcium behavior in soil
- Rapid equilibration between exchangeable calcium and soil solution calcium
- Exchangeable Ca2+ buffers the solution Ca2+ pool
- Ca2+ has slight mobility in soil solution
- Soil solution concentration of 15 ppm Ca sufficient for most crops
- Soil solution concentrations between typically 30 and 300 ppm Ca in temperate region soils
- Ca2+ held more strongly than most cations
- Al3+ > Ca2+ > Mg2+ > K+ = NH4+ > Na+
- Cation exchange reactions dominate calcium behavior in soil
- Calcium availability
- Calcium less available at low pH
- Low Ca2+ saturation percentage of exchange sites
- Solution aluminum (Al3+) inhibits Ca2+ uptake
- Effect of other ions
- Excessive levels of NH4+, Mg2+, K+, can inhibit Ca2+ uptake
- Calcium less available at low pH
- Calcium transport
- Erosion
- Losses from high CEC soils can be large
- Leaching
- Does not normally occur to any appreciable extent because of relatively strong attraction to clay particle surfaces
- Calcium often the dominant cation in water percolating through the soil
- Calcium removal by leaching is factor in long-term soil acidification
- Erosion
¶ Figure 2. Calcium Sources
¶ C. Calcium deficiency conditions
- Deficiency affected by widely varying presence of calcium minerals in soil
- Lower concentrations more common in coarse-textured than fine-textured soils
- Much calcium has been removed in highly weathered soils
- Moderate levels in many humid, temperate regions
- Surface soil may be acidic with low calcium
- Subsoil may have adequate calcium
- Highest in calcareous soils
- Due to presence of calcium carbonates
- More common in semi-arid to arid regions than more humid regions
- Gypsum minerals (CaSO4) most common in semi-arid and arid regions
- Sensitive crops
- Tree crops: almonds, apples
- Vegetables: peppers, tomatoes, cabbage, carrots
- Peanuts: large seeded Virginia-type peanuts require more calcium than runner and Spanish types
- Deficiency conditions
- Sandy, low CEC soils with high leaching conditions
- Poor growth usually due to excess soluble aluminum, manganese, and/or iron rather than inadequate calcium
- Crops that require low pH
- Controlling scab in potatoes
- Crops with high calcium requirements or high translocation needs
- Sandy, low CEC soils with high leaching conditions
- Physiological disorders
- Often not a soil fertility problem
- May be distribution (translocation) problem within plant
- Inadequate calcium supply to tissue with low transpiration rates, like fruits, young leaves
- For example, fluctuations in soil moisture, heavy applications of nitrogen fertilizer, or root injury can predispose tomato plants to blossom end rot
- Often not a soil fertility problem
- Water management
- Deficiency can be aggravated by alternating wet/dry conditions
- Continuous calcium uptake is required
- Irrigation management can be important
¶ D. Soil Testing for Calcium
- Usually expressed as “exchangeable Ca” concentration
- Includes both water soluble and exchangeable forms
- Ca2+ ions on exchange sites displaced by cation in extracting solution
- Analytical methods
- Ammonium acetate (1N, pH 7.0) extraction
- Uses NH4+ as extracting cation
- May include some non-exchangeable calcium in calcareous soils (high excess lime)
- Dissolved from calcium carbonates
- May inflate CEC calculation
- Mehlich-3 (pH 2.5) extraction
- Uses NH4+ as extracting cation
- Includes non-exchangeable calcium in calcareous soils (low to high excess lime)
- Dissolved from calcium carbonates
- Other methods may use NH4+ or Na+ as extracting cation
- Concentrations in filtered extract determined by atomic absorption spectroscopy (AAS) or inductively coupled plasma spectrometry (ICP)
- Ammonium acetate (1N, pH 7.0) extraction
- Critical levels
- Common critical range for sandy soils from 200 to 300 ppm exchangeable Ca
- Common critical range for silty, clayey, and organic soils from 250 to 500 ppm Ca
- Peanuts: Above 600 to 800 ppm Ca
- Calcium saturation
- Calcium considered “basic” cation
- Exchangeable Ca concentration used in calculations for cation exchange capacity (CEC) and base saturation
- % Ca saturation = [(Ca ppm / 200) / CEC as mEq per 100g] * 100
- Calcium carbonate dissolution has minor effect on CEC calculation if less than 4000 to 5000 ppm Ca
- Wide range of calcium-to-magnesium ratios have no effect on plant growth as long as soils are above critical levels
¶ E. Calcium Nutrient Management
- Organic, biological sources
- Organic matter
- Much of the calcium is readily leached from crop residues
- Most of the remainder is mineralized during early stages of residue decomposition
- Manure, compost, biosolids
- Manure content varies widely
- Ranges from 5 to more than 50 lb Ca per wet ton
- Depends on animal diet, collection strategy , storage methods
- Most calcium is in soluble, readily available form
- Manure content varies widely
- Organic matter
- Inorganic fertilizer materials
- Liming materials
- Most calcium applied to soil is in materials used to neutralize soil acidity
- Calcitic limestone, CaCO3
- Primarily contains calcium carbonate
- Common analysis: calcium content varies, but has maximum of about 40% Ca
- Dolomitic limestone, MgCO3•CaCO3
- Contains significant amounts of magnesium carbonates
- Common analysis: 24% to 30% Ca, 6% to 20% Mg
- Gypsum (CaSO4)
- Supplies calcium without affecting pH
- Common analysis: 22% Ca, 18% S
- Calcium nitrate, Ca(NO3)2
- Very soluble
- Typically applied as foliar spray or in irrigation water
- Common analysis: 19% Ca, 15.5% N
- Calcium chloride, CaCl2
- Very soluble
- Typically applied as foliar spray
- Common analysis: 29% to 36% Ca
- Also contained in fertilizers supplying other nutrients
- Single superphosphate: 18% to 21% Ca, 16% to 20% P2O5, 13% to 16% N
- Triple superphosphate: 15% Ca, 45% P2O5
- Irrigation water
- Each 1 mg/L Ca in water equivalent to 0.23 lb Ca per acre-inch applied as irrigation
- Liming materials
- Rates and placement
- Soil calcium generally managed through liming
- If soil pH is in desired range, calcium is usually adequate for most crops
- Recommended calcium rates based on “build” approach
- One-time application to increase existing soil test level past critical level into optimum level
- Recommended application usually considered adequate for following three to five years
- Soil calcium generally managed through liming
- Calcium foliar sprays
- Usually applied to high value crops
- May be helpful for storage disorders
- Application must reach the affected tissue
- Must apply to point of runoff
- Foliar fertilizer solutions commonly include Ca(NO3)2, CaCl2, or chelate materials
- Sprays or dips may improve storage life of harvested fruits
- Usually applied to high value crops