⇦ Back to Soil Fertility and Plant Nutrition Home
¶ Introduction
Potassium transformations are less about the chemical form of potassium and more about the relationship of the potassium ion with various soil minerals.
¶ A. Primary soil transformations
- Weathering of primary soil minerals
- Feldspars, micas are main primary potassium minerals
- Breakdown of crystal structure releases some potassium to soil solution
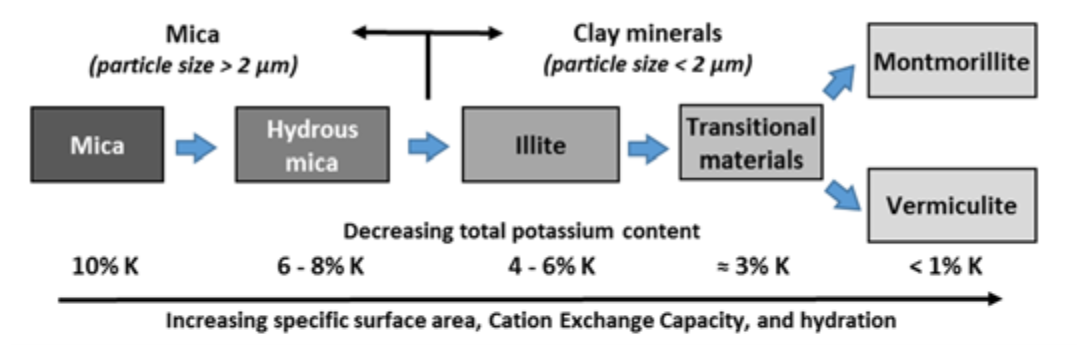
- Primary minerals are altered by weathering into various types of secondary clay-fraction minerals (see Fig. 1)
- Duration and type of weathering affects type of secondary clay minerals that result
- Cation exchange
- Potassium ion (K+) adsorbs ↔ desorbs from clay and organic matter exchange surfaces
- Most important potassium reaction in soil
- Influenced by clay mineralogy characteristics
- Fixation
- Nonexchangeable, interlayer potassium
- Involves secondary clay minerals
- Fixation ↔ release
- Greatly affected by clay mineralogy
- Nonexchangeable, interlayer potassium
¶ Figure 1. Generalized Mineral Weathering Process
¶ B. Mineral weathering
- Earth’s crust averages about 2.6% total potassium
- About 95% to 99% of potassium contained in feldspar and mica minerals
- Feldspar minerals
- Microcline, KAlSi3O8
- Orthoclase, KAlSi3O8
- Mica minerals
- Muscovite, H2KAl3(SiO4)3
- Biotite, (H,K)2(Mg,Fe)2Al2(SiO4)3
- Mineral weathering supplies significant amounts of potassium in some soils
- Differing potassium content of primary minerals that make up parent materials affect clay minerals
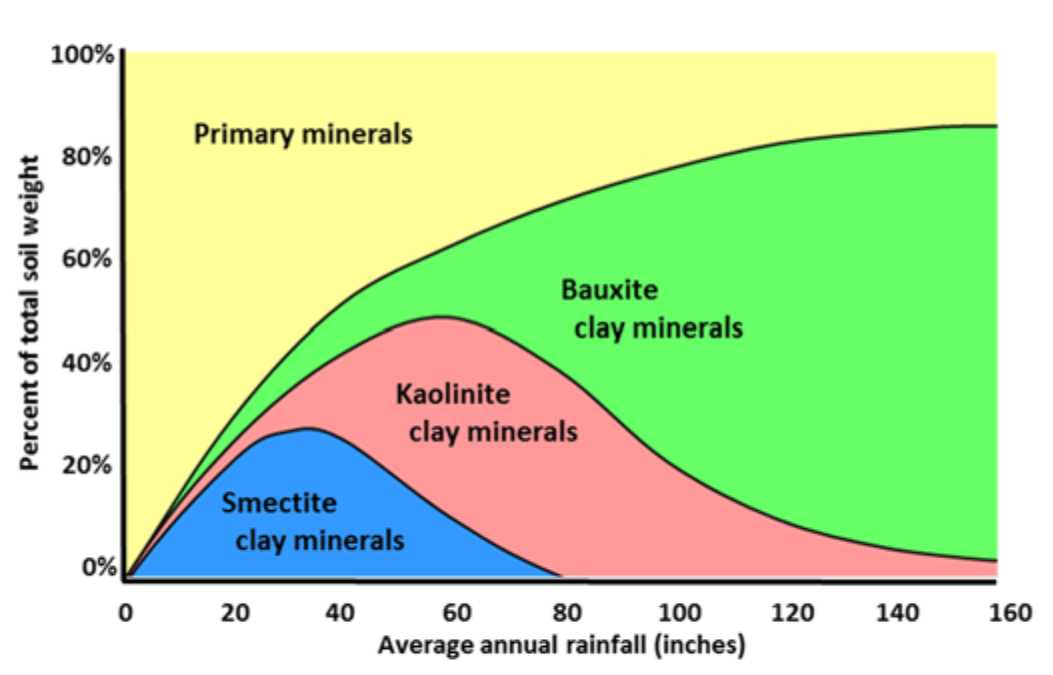
- Intensity of weathering depends on environment, especially on rainfall
- Degree of weathering affects clay mineral development (see Fig. 2)
- Heavy, continuous weathering occurs in high rainfall, tropical regions
- Very little weathering occurs in low rainfall, desert areas
- Potassium release to available K+ affected by weathering intensity and primary mineral characteristics
- Feldspar minerals weather slower than mica minerals
- Feldspars tend to exist as larger soil particles, i.e. sand-sized fractions
- Micas tend to exist as smaller particles, i.e. silt-sized and clay-sized fractions
- Feldspar minerals release potassium much less readily than mica minerals
- Potassium is held in crystalline structure of feldspars
- Weathering releases potassium ions (K+) from feldspar crystals into to soil solution
- Weathering provides largest fraction of potassium content in moderately weathered soils
- Is only small fraction of total potassium content in highly weathered soils
- Micas are 2:1 layer silicates with potassium in interlayer positions
- Weathering progressively forms secondary 2:1 clay minerals
- e.g., mica → illite → vermiculite
- Potassium is held in crystalline structure of feldspars
¶ Figure 2. Weathering and Transformation of Basalt Primary Minerals in Hawaii
¶ Table 1. Comparative Properties of Three Major Clay Types |
|||
|---|---|---|---|
| Property | Montmorillonite | Illite | Kaolinite |
| Surface area (m 2 /g) | 700 ‐ 800 | 100 – 200 | 5 – 20 |
| External surface area | High | Medium | Low |
| Internal surface area | Very High | Medium | None |
| Cohesion ("stickiness" ) | High | Medium | Low |
| Swelling capacity | High | Medium | Low |
| Cation exchange capacity (mEq/100g ) | 80 – 100 | 15 – 40 | 3 – 15 |
¶ C. Secondary clay mineral characteristics
- Clay particles are “laminated”
- Individual clay particles made up of layers or plates
- Layers of silicate clays have both silicon and aluminum in clay chemical structure (“aluminosilicate”)
- Typical of temperate regions
- Four predominant classes based on number of layers and characteristics
- Iron/aluminum oxide minerals typical of tropics and semi-tropics may form crystalline sheets
- May also form coatings on clay particles
- All clay types have exposed external surfaces; some have extensive internal surfaces
- External and internal surfaces can both provide cation exchange sites
- Clays have about 1000 times as much surface area as same weight of coarse sand
- All clays have water molecules intermixed with cations on external exchange surfaces
- Some clays can hold numerous water molecules and cations between layers
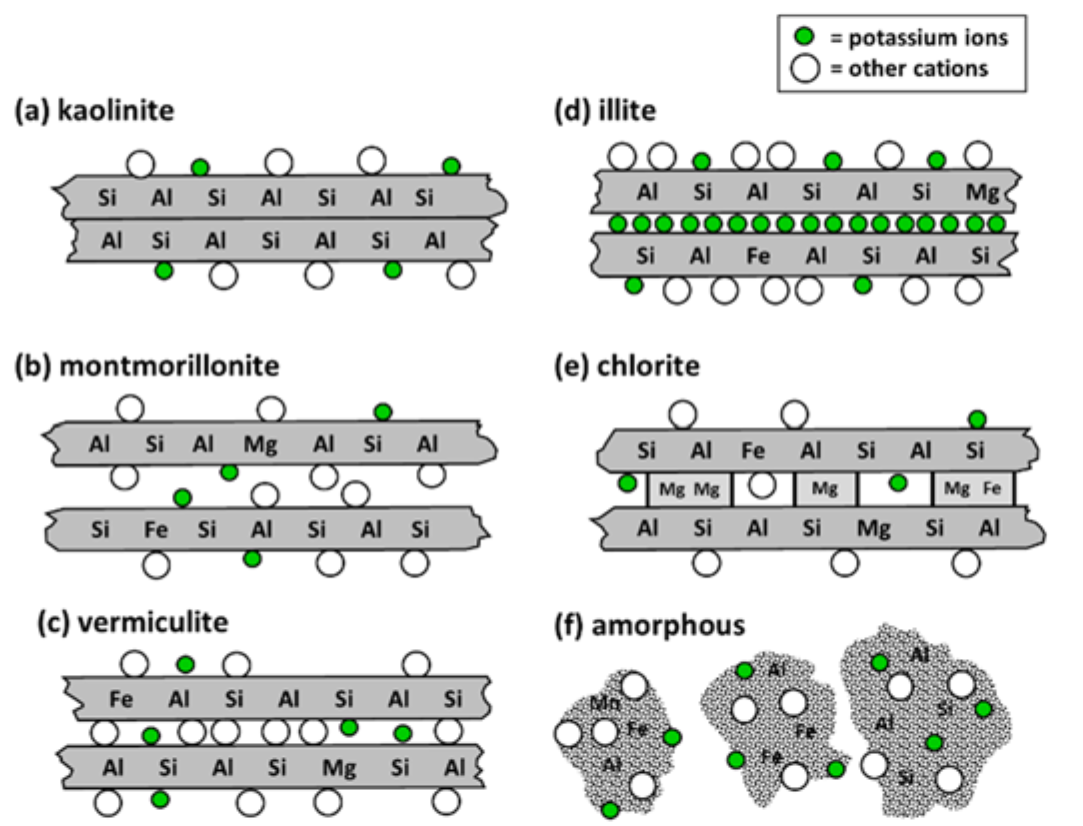
- 1:1 non-expanding clay minerals
- Kaolinite is predominant type in soils
- Most highly weathered clay type
- One silica layer plus one alumina layer (see Fig. 3(a))
- No internal surface area
- Have low capacity to adsorb cations (see Table 1)
- Kaolinite is predominant type in soils
- 2:1 expanding clay minerals
- Montmorillonite and vermiculite are common 2:1 expanding clays
- One alumina layer sandwiched between two silica layers (see Fig. 3(b), Fig. 3(c))
- Have large internal surface area compared to external surface area
- Greater total surface area compared to kaolinite
- These minerals lack interior potassium that can bind layers together
- Cations attracted to internal surfaces
- Water molecules and hydrated ions (ions with “coating” of water molecules) force layers apart
- Lack of binding allows clay particle to swell when wet, to shrink when dry
- Montmorillonite
- Montmorillonite is common clay; member of smectite group of clay minerals
- Montomorillonite clay particles smaller than kaolinite particles
- Much greater shrink-swell potential than vermiculite
- iiv. Are highly cohesive (“sticky”) clays (see Table 1)
- iv. Have high plasticity (high pliability, capacity to be molded)
- Vermiculite
- “Limited expansion” clay type
- Less internal surface than montmorillonite
- Greater cation absorption (CEC) than montmorillonite
- Montmorillonite and vermiculite are common 2:1 expanding clays
- 2:1 non-expanding minerals
- Illite clays are most important member of this group in agricultural soils (see Fig. 3(d))
- Internal surfaces strongly attract potassium
- Strong potassium bonds prevent layers from expanding
- Cation exchange capacity, shrink-swell potential, and cohesion lower than montmorillonite (see Table 1)
- 2:1:1 type clay minerals
- Chlorite group represent these clays (see Fig. 3(e))
- Complex clays; consists of silicate sheets with magnesium minerals in interlayer space
- Particle size and surface area similar to illite
- Cation exchange capacity similar to illite; much lower than montmorillonite or vermiculite
- Does not absorb water; considered partially-expanding to non-expanding clay
- Chlorite group represent these clays (see Fig. 3(e))
- Amorphous clay minerals (see Fig. 3(f))
- Amorphous = non-crystalline clay-sized particle
- Often have high specific surface area, 900 to 1100 m2/g, compared to other clays (see Table 1)
- Can adsorb both cations and anions
- Mostly adsorb cations at high pH
- Mostly adsorb anions at low pH
- Allophanes
- Amorphous (“structurally disordered”) aluminosilicate mineral, Al2O3• 2SiO2•H2O
- Weathered from volcanic glass (volcanic ash) or feldspar
- Often associated with kaolinite clays
- Sesquioxides
- Clay-sized metal oxides and hydrous oxides (oxides containing associated water molecules)
- Widely varying chemical composition
- Typically found in highly weathered soils (i.e., tropics, subtropics)
- Intermixed with silicate clays
- Are formed from clays as silica is depleted by leaching
- Hydrous oxides of aluminum, iron, and manganese are common types
- Gibbsite, Al2O3•xH2O
- Goethite, FeO(OH)•xH2O
- Hematite, Fe2O3•xH2O
- Birnessite, Mn2O4•xH2O
- Clay-sized metal oxides and hydrous oxides (oxides containing associated water molecules)
- Amorphous = non-crystalline clay-sized particle
- Mixed layer
- Clay mineral groups do not occur independently of each other
- Individual soil may contain mixture of several clay mineral groups
- Individual particles may have characteristics intermediate between two groups
- e.g., “chlorite-illite” or “illite-montmorillonite”
- Intermediate clays may be more common than single structure clays
¶ Figure 3. Representative Clay Mineral Structures
¶ D. Cation exchange
- Cation exchange reactions dominate soil potassium behavior (see Crop File 1.04.410, Potassium in the Soil and Plant)
- Adsorption ↔ desorption from clay and organic matter surfaces
- Monovalent potassium ion (K+) held less strongly than polyvalent cations, but more strongly than other monovalent cations
- Al3+ > Ca2+ > Mg2+ > K+ = NH4+ > Na+
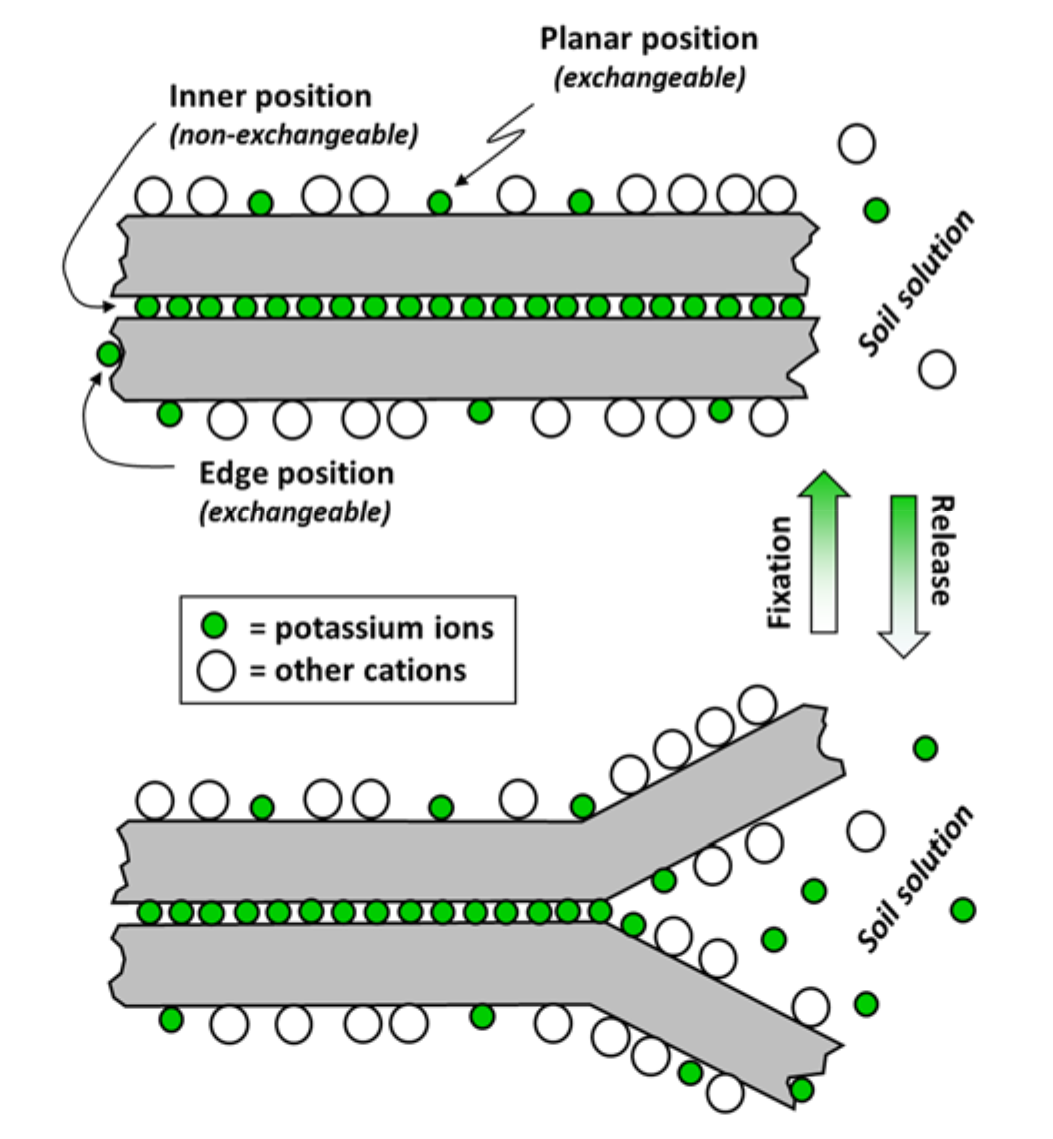
- Clay types may have different potassium exchange sites (see Fig. 4)
- Planar position sites
- Cations adsorb to external surfaces of clay minerals
- These sites not specific for potassium; available as sites for other cations
- Adsorption bond is comparatively weak; potassium can be “easily” replaced by other cations
- Edge position sites
- Clay mineral edges
- Sites are highly specific for potassium
- Bond is not very strong
- Inner position sites
- Inner surfaces of clay minerals
- Sites are very specific for potassium
- Bond is very strong, sites not readily available for exchange with other cations
- Planar position sites
- Concentration of solution potassium vs. concentration exchangeable potassium affected by:
- Kinds and amounts of other cations
- Nature of soil exchange sites
- Rapid equilibration occurs between exchangeable potassium and solution potassium
- Potassium ions (K+) on external exchange surfaces (planar positions) most readily buffer potassium ions (K+) in soil solution
- Equilibrium affected by quantity and intensity, but there is no simple relationship between them
- Affected by base saturation and pH
- Base saturation: ratio of base cations (Ca2+, Mg2+, K+, and Na+) to H+ plus Al3+ on soil exchange sites expressed as percent
- Base saturation percentage increases if H+ and Al3+ on exchange surfaces are displaced by Ca2+and Mg2+
- At pH range of 5 to 6, each 0.10 change in pH changes base saturation by 5%
- K+ ion replaces Ca2+ and Mg2+ ions much more readily than it replaces Al3+
- Solution K+ ions may be adsorbed onto newly available exchange sites
- Solution potassium may decrease, exchangeable potassium may increase if cations are replaced
¶ E. Fixation
- Non-exchangeable potassium
- Not immediately available, but in equilibrium with labile potassium sources
- Soil solution K+ ← (rapid) → exchangeable K+
- Exchangeable K+ ← (slow) → non-exchangeable K+
- Not immediately available, but in equilibrium with labile potassium sources
- "Fixed" potassium is held tightly in interlayer positions
- Potassium ion (K+) fits between layers of clay minerals
- Limits expansion of layers (low shrink-swell)
- Is essentially “unavailable” to plants
- Does not readily exchange with solution potassium
- Layered clay sheets may “unzip” at edges exposing internal surfaces to release potassium (see Fig. 4)
- Released potassium can be trapped again later between layers as edges “zip” close
- Potassium ion (K+) fits between layers of clay minerals
- Potassium fixation is slowly reversible process
- Fixed potassium may be released to maintain equilibrium when exchangeable potassium is depleted
- May be released when environmental conditions change (e.g., wet/dry cycles)
- Layered clay may be “unzipped” to expose interlayer surfaces
- Potassium ion (K+) migrates out of interlayer spaces
- Can be held along edges of particles undergoing weathering
- Ions then released into soil solution via cation exchange
- Factors affecting potassium fixation and release
- Amount and type of clay
- Higher clay content, higher fixation potential
- Most prominent in fixation process are montmorillonite, vermiculite, and weathered micas (i.e., layered clays)
- Net effect of fixation/release depends on dominant clay mineral In mixed clay systems
- e.g., soil containing both montmorillonite and illite
- Less potassium is fixed by montmorillonite when Fe2+ → Fe3+ during drying
- More potassium is fixed by illite when Fe2+ → Fe3+ during drying
- Presence of ammonium (NH4+)
- Ammonium ion (NH4+) about same size as potassium ion
- Ammonium ions can be “fixed”, like potassium
- Ammonium ion can fit into interlayer positions, trapping potassium ions
- Wet/dry cycles
- Exchangeable potassium can increase or decrease when soil is dried
- Depend on clay minerals present
- Net effect depends on whether fixation or release dominates
- Fixation can occur when some soils are dried
- Soils with high exchangeable potassium or recent fertilizer application
- Result of potassium ions becoming trapped within clay sheets as they dry and collapse
- Release can occur when some soils are dried
- Soils low in exchangeable potassium
- Clay sheets roll back and release K+ ions
- Freeze/thaw cycles
- Effects vary with exchangeable potassium levels, clay minerals, winter weather
- Soils with considerable amount of mica: fixed potassium can released
- Soils with smaller amounts of mica and greater amounts of exchangeable potassium: release/fixation not impacted
- Soil test potassium levels may be different in spring vs. fall due to winter weather
- Liming
- Lime removes H+ ions from interlayer surfaces
- Provides more inner layer sites for “storage” of non-exchangeable K+ ions
- Exchangeable potassium can increase or decrease when soil is dried
- Amount and type of clay
- Fixation of fertilizer potassium
- Potassium ions (K+) from fertilizer can move into 2:1 clay interlayer sites
- Not quick process; may take several months
- Fertilizer potassium may be readily available immediately after application
- Potassium availability may decline over time
- Different soils may have different proportions of potassium fixing minerals
- Identifying clay mineralogy requires complicated lab analysis
- Certain clay groups are typically associated with certain soil groups (see Crop File 1.04.412, Great Soil Orders and Associated Clay Minerals)
- Nutrient management
- Use smaller, more frequent applications on soils with high potassium fixation capacity
- Broadcast and incorporating
- Least desirable choice for fine-textured soils with high fixation potential
- Band placement minimizes contact between soil and fertilizer
- Can limit potassium fixation potential
- Most beneficial on low potassium soils with high fixation capacity
¶ Figure 4. Layered Clay "Unzipping" Due to Wet/Dry Cycle; Potassium Fixation <—> Potassium Release