⇦ Back to Soil Fertility and Plant Nutrition Home
¶ Potassium or Potash?
Potassium (K) is a soft, silvery-white alkali metal, but occurs in nature only as an ionic salt. “Potash” can refer to any of several compounds containing potassium. The name was derived from “pot ash”, which refers to wood ashes soaked in water in a pot, resulting in an impure form of potassium carbonate. “Pot ash” was used in making soap, bleaching textiles, and making glass. The potassium content of commercial fertilizers is commonly expressed as an oxide form, “K2O”. (K x 1.16 = K2O, K2O x 0.86 = K)
¶ A. Potassium in the Plant
- Considered primary macronutrient
- Potassium requirements second only to nitrogen
- Sufficiency range of recently mature leaves typically 1.5% to 4% on dry weight basis
- Highest potassium concentrations found in new leaves, petioles, stems, and ruit
- Little potassium found in grain
- Plant tissue potassium concentration closely related to nitrogen concentration in most plants
- N:K ratio (nitrogen-to-potassium ratio) often 1:1
- Nitrogen stimulates rapid, soft tissue growth
- Potassium balances effect by promoting growth of firmer tissues
- Potassium requirements second only to nitrogen
- Major functions
- Activates enzymes involved in:
- Starch synthesis
- ATP production
- Photosynthesis
- Nitrate reduction
- Translocation of sugars to grain, fruit, tubers, roots
- Cell water relations
- Affects ionic strength of cytoplasmic solution
- K+ controls cell water potential and osmosis
- Na+ can substitute for much of the K+ requirement in some species
- Cell turgor
- Plant rigidity
- Opening and closing of stomata
- Water uptake by roots
- Osmotic suction
- Potassium and stress resistance
- Helps control of water loss by transpiration and water absorption by roots
- Helps maintain stem moisture until maturity and harvest
- Results in:
- Increased resistance to infection
- Greater drought tolerance
- Increased tolerance to insect feeding
- Reduced lodging
- Improved winter-hardiness
- Activates enzymes involved in:
- Mobility within plant
- Potassium exists only in ionic form
- Not incorporated into the structure of organic compounds
- K+ is in solution (e.g., cell contents) or bound to negative charges on tissue surfaces
- Potassium highly mobile in plant
- Translocated from older leaves to young growing points
- Potassium exists only in ionic form
- Deficiency symptoms
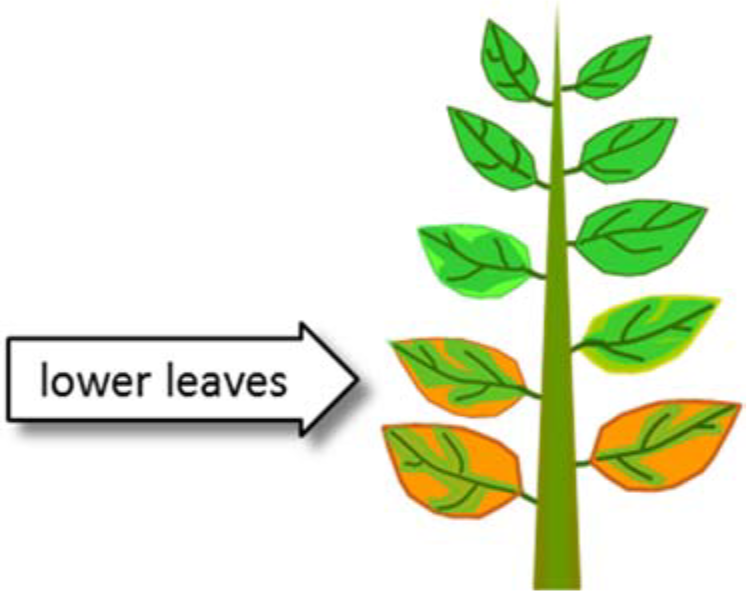
- Chlorosis of leaf tips and margins
- Scorch: necrosis of leaf tips and margins
- Begins on older, lower leaves
- Legumes
- White/necrotic spotting on leaflet margins
- Can be mistaken for insect feeding
- Legumes
- Increased lodging, winter injury, drought stress, reduced disease and insect resistance
- Chlorosis of leaf tips and margins
- Excess potassium
- Not directly toxic to plants or other organisms
- May be component of excess soil salinity
- Marginal foliar burning from high salts usually affects younger leaves first
- High soil potassium can inhibit uptake of other cations
- May induce magnesium or calcium deficiency
- Potassium can counteract problems of excess nitrogen
- Nitrogen stimulates vegetative growth
- Nitrogen increases proportion of soft, succulent plant tissues
- Excess nitrogen increases susceptibility to lodging, disease infection, insect damage
- Potassium has effect opposite to nitrogen
- Not directly toxic to plants or other organisms
¶ Figure 1. Potassium Deficiency Symptoms
¶ B. Potassium in the Soil
- Potassium uptake
- Taken up by roots as K+ cation
- K+ moves by both diffusion and mass flow
- Soil solution concentrations between 1 and 10 ppm K
- Average about 4 ppm K in agricultural soils
- Distance of potassium movement is very limited
- Only 1 to 4 mm during growing season
- Diffusion accounts for about 85% to 95% of root absorption in many soils
- Affected by moisture content, temperature, tortuosity of diffusion path through soil
- Root surface area is important to absorption (root radius, length, growth rate)
- Uptake through mass flow accounts for about 10% of plant potassium requirement
- More important in very high potassium soils or recently fertilized soils
- Mass flow more important in low CEC soils; are fewer cation exchange sites
- “Luxury” consumption
- If soluble K+ is very high, some plants will take up much more potassium than needed for optimum growth
- Elevates plant potassium content with no effect on yield, either positive or negative
- May lead to excess potassium content and removal in harvested products
- Elevated potassium content in harvested products can cause nutritional imbalances in livestock
- e.g., High potassium forages (> 2.5% K) associated with milk fever in dairy cows
- Taken up by roots as K+ cation
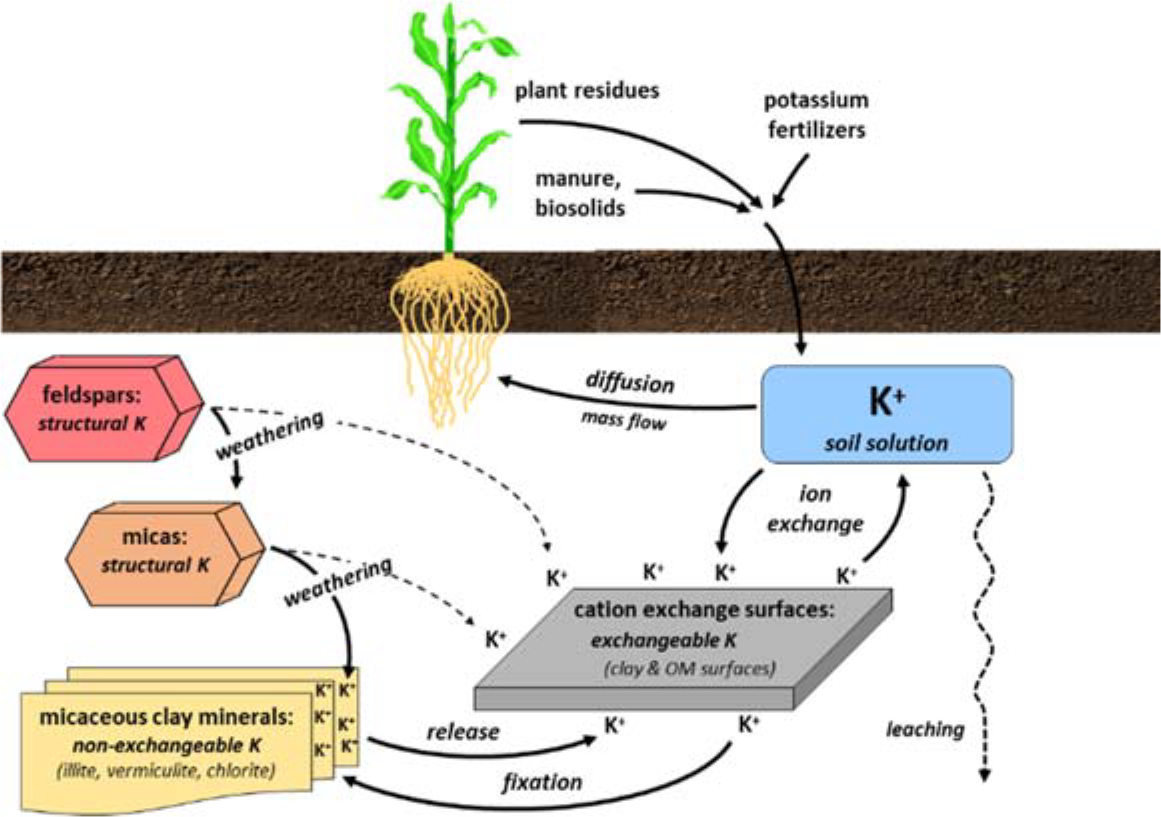
- Soil transformations
- Cation exchange reactions
- Adsorption ↔ desorption from clay, organic matter exchange surfaces into soil solution
- K+ is an exchangeable cation
- Most important soil potassium reaction
- Adsorption ↔ desorption from clay, organic matter exchange surfaces into soil solution
- Fixation ↔ release
- Potassium ions can be strongly complexed in interlayer regions of clay mineral structure
- Fixation potential affected by type and proportion of secondary clay minerals in soil material
- Vermiculite, illite, and chlorite clays: high fixation potential
- Smectite, montmorillonite clays: low fixation potential
- Weathering of primary soil minerals to release potassium into soil solution
- Predominantly feldspars and mica minerals
- Affected by both soil temperature and soil moisture
- Reactions slower in cool/cold and dry soils
- Conditions for root growth affect uptake
- Transient deficiencies may occur in high potassium soils
- Cation exchange reactions
- Potassium availability to roots
- Readily available potassium
- Soil solution plus exchangeable potassium
- Labile fractions: readily reactive, readily changed to available forms
- Less than 1% to 2% of total soil potassium
- Soil solution plus exchangeable potassium
- Slowly available potassium
- Nonexchangeable, “fixed” potassium
- Non-labile fractions: react slowly in soil
- Held within clay layers by strong bonds
- About 1% to 10% of total soil potassium
- Nonexchangeable, “fixed” potassium
- Unavailable potassium
- Potassium as part of primary mineral structure
- Very slowly replenishes available potassium
- Non-labile fractions: nearly unreactive
- About 90% to 98% of total soil potassium
- Readily available potassium
- Sources of soil solution potassium
- Weathering (dissolution) of primary potassium-containing minerals
- Many soils have large amounts of total potassium as part of mineral structure
- Most soils have more total potassium than any other nutrient
- Common soil concentration: 5,000 to 25,000 ppm K
- Feldspars and mica minerals are primary potassium minerals
- Micas: 8% to 10% potassium content
- Feldspars: 12% to 14% potassium content
- Primary minerals are long-term potassium source
- VERY slowly available - over period of years or decades
- Considered to be agronomically unavailable for current crop season
- Many soils have large amounts of total potassium as part of mineral structure
- Nonexchangeable potassium
- Potassium ions (K+) found in interlayer positions of some groups of 2:1 clay minerals
- K+ ion fits well in molecular “holes” inside of certain clay minerals
- Notably illite, vermiculite, chlorite clays
- Common soil concentration: 50 to 750 ppm K
- Potassium ions (K+) found in interlayer positions of some groups of 2:1 clay minerals
- Exchangeable potassium
- K+ ion exchanges with clay, organic matter surfaces
- Common soil concentration: 40 to 600 ppm K
- Organic matter
- Majority of potassium is readily leached from crop residues
- Potassium release does not depend upon organic material decomposition (mineralization) as do nitrogen or phosphorus
- Manure, compost, biosolids
- Most potassium is in organic forms, so is soluble, readily available
- Depends on “ash” content (i.e., dirt, sand, etc.)
- Potash fertilizers
- Potassium is in soluble, readily available forms
- Weathering (dissolution) of primary potassium-containing minerals
- Soil potassium buffering
- Intensity
- Soil solution potassium concentration
- Immediately available potassium
- Capacity
- Quantity of exchangeable potassium sources
- Is in rapid equilibrium with solution potassium
- Replaces potassium taken up by roots
- Buffering capacity
- Ability of solid forms of soil potassium to replenish solution potassium taken up by plant roots
- Is ability of soil to maintain nutrient concentrations in soil solution
- Affected by both “intensity” and “capacity”
- Buffering capacity proportional to CEC
- Soil tests measure solution potassium and exchangeable potassium
- Some nonexchangeable (fixed) potassium may also be released rapidly enough to become available during a growing season
- Ability of solid forms of soil potassium to replenish solution potassium taken up by plant roots
- Intensity
- Fate of fertilizer potassium
- Fertilizer potassium is very soluble, will increase solution potassium concentrations
- Added potassium is subject to cation exchange and possibly to some degree of fixation
- Soils with high buffering capacity
- Will remove some fertilizer potassium ions from solution
- Intensity (solution potassium concentration) of highly buffered soil may be much lower than intensity of low CEC soils
- Highly buffered soils may have lower initial concentration, but have greater capacity to maintain solution potassium than do poorly buffered soils
- Potassium uptake affected by presence of other cations
- Solution activity ratios are better estimate of availability
- Activity of K+ / (activity of Ca2+ + activity of Mg2+)½
- May also need to consider Al3+ in acid soils and Na+ in salt-affected soils
- Solution activity ratios are better estimate of availability
¶ Figure 2. Potassium Sources
¶ C. Soil Testing for Potassium
- Soil test provides index of relative buffering ability
- Extracting solution and soil in contact for only short time (e.g., five minutes)
- Soil tests “measure” solution potassium and exchangeable potassium
- Amount of potassium extracted during extraction relates to soil’s ability to resupply soil solution potassium over entire growing season
- Test methods intended to provide estimate of comparative season-long “availability”
- Soil test must be calibrated to growth response or yield response by appropriate field research
- Method mode of action
- Cation replacement: Selected cation targets potassium adsorbed to cation exchange surfaces
- Adsorbed potassium cation (K+) in soil sample is replaced by cation supplied in extracting solution
- Soil:solution mixture is filtered, removing soil from solution
- Instruments determine potassium ion content in filtered solution
- Common testing methods
- Methods typically use NH4+ or Na+ as extracting cation
- Ammonium acetate (1N, pH 7.0) solution
- Uses NH4+ as extracting cation
- May inflate CEC value in calcareous soils
- Mehlich-3 (pH 2.5)
- Uses NH4+ as extracting cation
- May inflate CEC value in calcareous soils
- Olsen bicarbonate (0.5 M N aHCO3, pH 8.5)
- Uses Na+ as extracting cation
- Sodium tetraphenylboron
- Used to estimate non-exchangeable (fixed), plant-available potassium
- Critical soil analysis levels
- Analysis result expressed as “exchangeable K”
- Most agronomic crops: 120 - 150 ppm K
- Grasses & small grains: 80 - 120 ppm K
- Vegetables & ornamentals: 120 - 200 ppm K
- Potassium saturation
- Potassium considered “basic” cation
- Exchangeable K concentration used in calculations for cation exchange capacity (CEC) and base saturation
- % K saturation = [(K ppm / 390) / CEC mEq/100g] * 100
- Wide range of potassium/calcium/magnesium ratios have no effect on plant growth as long as soils are above critical levels
¶ D. Potassium Nutrient Sources
- Fertilizer potassium content expressed as “percent potash” or “% K2O”
- Potassium not actually present in fertilizers as K2O
- Holdover from 1800’s when chemists expressed elemental concentrations in “oxide” form
- Many original laws governing fertilizer guarantees were written during this time
- Organic, biological sources
- Manure
- Common analysis, dried manure: 1% to 2% K2O
- Depends on storage and treatment method
- Biosolids, compost
- Common analysis: 0.5% K2O or less
- Depends on storage and treatment method
- Wood ashes
- Common analysis: 3 to 7% K2O, 1% to 2% P2O5
- Type of impure potassium carbonate
- Can be used as liming material
- Manure
- Common inorganic fertilizer materials
- Potassium chloride, KCl
- Common analysis: 0-0-60 or 0-0-62, 45% to 47% Cl
- Common trade name: muriate of potash (MOP)
- Red or white solid granular material
- High water solubility
- Most widely used potassium fertilizer
- Potassium-magnesium sulfate, 2MgSO4•K2SO4
- Common analysis: 0-0-22, 18% magnesium, 22% sulfur
- Common trade names: sulfate of potash-magnesia, K-Mag, Sul-Po-Mag
- Granular product manufactured from langbienite
- Totally soluble, but slower to dissolve than other sources
- Potassium thiosulfate (KTS), K2S2O3
- Common analysis: 0-0-25, 17% sulfur
- Clear liquid product
- Major use: adding sulfur to starter fertilizer materials and UAN solutions (e.g., 28-0-0)
- Use caution when blending with UAN solution; may form KNO3 crystals
- Not recommended for direct seed placement
- Potentially damaging to seedling root and shoot tissues
- Also used for fertigation or as foliar fertilizer
- Potassium sulfate, K2SO4
- Common analysis: 48% to 53% K2O, 17% to 18% S
- Common trade name: sulfate of potash (SOP)
- Water soluble granular product
- About one-third less soluble than KCl
- Salt measurement (EC) from K2SO4 solution is less than third of similar KCl solution
- Potassium nitrate, KNO3
- Common analysis: 48% to 53% K2O. 17% to 18% S
- Common trade name: nitrate of potash (NOP)
- Water soluble granular material; primarily used to make solution fertilizers
- Chloride-free fertilizers required by some plants
- Potassium carbonate, K2CO3
- Common analysis
- Solid material: 0-0-48
- Liquid material: 0-0-34
- Used to manufacture clear, mixed-liquid fertilizers
- Common analysis
- Potassium acetate, CH3CO2K
- Common analysis: 0-0-29
- Liquid material produced by combining potassium hydroxide (“lye”) with acetic acid (“vinegar”)
- Used to manufacture clear, mixed-liquid fertilizers
- Potassium chloride, KCl
¶ E. Potassium Nutrient Management
- Potassium has limited mobility in soil
- Activity of potassium ion lower in cold and dry soils with high potassium soil test
- Transient deficiencies may occur, disappearing with improved growing conditions
- Placement
- Topdress (surface broadcast, no incorporation)
- Potassium will move to roots very slowly
- Acceptable for alfalfa, perennial forage grasses, turf grasses
- Surface broadcast with incorporation
- Places potassium in root zone
- Less desirable method for fine-textured soils with high fixation capacity
- Band placement
- Minimizes contact between soil and fertilizer
- Most beneficial on low testing soils, especially those with high fixation capacity
- Concentrates soluble potassium in closer proximity to crop root system
- Placement with seed or too near seed increased potential for fertilizer “salt” injury
- Fertigation
- Proper material can be applied in sprinkler or drip systems
- Nitrate, chloride, or sulfate sources may be used or avoided depending on crop species
- Topdress (surface broadcast, no incorporation)
- Timing
- Single broadcast applications every three to four years are effective if fixation potential is low
- Smaller, more frequent applications beneficial on soils with high potassium fixation capacity
- Starter applications beneficial for early planted crops planted in cool or cold soils, poorly drained wet soils, or dry soils