⇦ Back to Soil Fertility and Plant Nutrition Home
¶ A. Mineralization
- Organic phosphorus released as plant available inorganic phosphate
- Factors affecting phosphorus mineralization from organic matter are generally same as those for nitrogen and organic matter
- Activity of bacteria, fungi, and actinomycetes
- Temperature
- Moisture, aeration
- Soil pH
- Nutrient content
- Residue: incorporation, particle size, surface area
- Phosphatase enzymes release orthophosphate ions
- Produced by a wide range of microbes
- Factors affecting phosphorus mineralization from organic matter are generally same as those for nitrogen and organic matter
- Organic phosphorus in soil
- Soil organic matter is about 1% phosphorus
- Up to 50% of organic forms are inositol phosphates
- Phospholipids, nucleic acids, less than 10% of organic phosphorus
- About 50% of organic phosphorus forms not well characterized
- Inositol phosphates
- Series of phosphate esters
- Inositol = C6H6(OH)6
- –OH- groups replaced by phosphate (PO4) groups
- Mostly phytic acid
- Inositol hexaphosphate (six phosphate groups)
- Products of microbial activity, residue decomposition
- Series of phosphate esters
¶ B. Immobilization
- Reverse of mineralization
- Uptake of inorganic phosphorus (HPO42- or H2PO4-) from soil and incorporation (assimiliation) into organic phosphorus forms by microbes
- Balance between mineralization and immobilization
- Carbon-to-phosphorus (C:P) ratio can limit organic matter decomposition in the same way as carbon-to-nitrogen (C:N) ratio
- Phosphorus mineralization can also be limited by C:N ratio
- C:P ratios vary more than C:N ratios
- C:P ratio of soil organic matter about 100:1
- C:N:P ratio of soil organic matter about 120:10:1.3
- High C:P ratio (low phosphorus content) may cause phosphorus immobilization
- Microbes use available phosphorus from soil solution
- Deplete supply available to plants
- When soil solution phosphorus is low:
- Microbial growth restricted
- Organic matter decomposition slows
- When C:P ratio greater than 300
- Residue less than 0.2% P
- Immobilization > mineralization
- When C:P ratio = 200 to 300
- Residue 0.2% to 0.3% P
- Immobilization = mineralization
- When C:P < 200
- Residue greater than 0.3% P
- Mineralization > immobilization
- Microbes use available phosphorus from soil solution
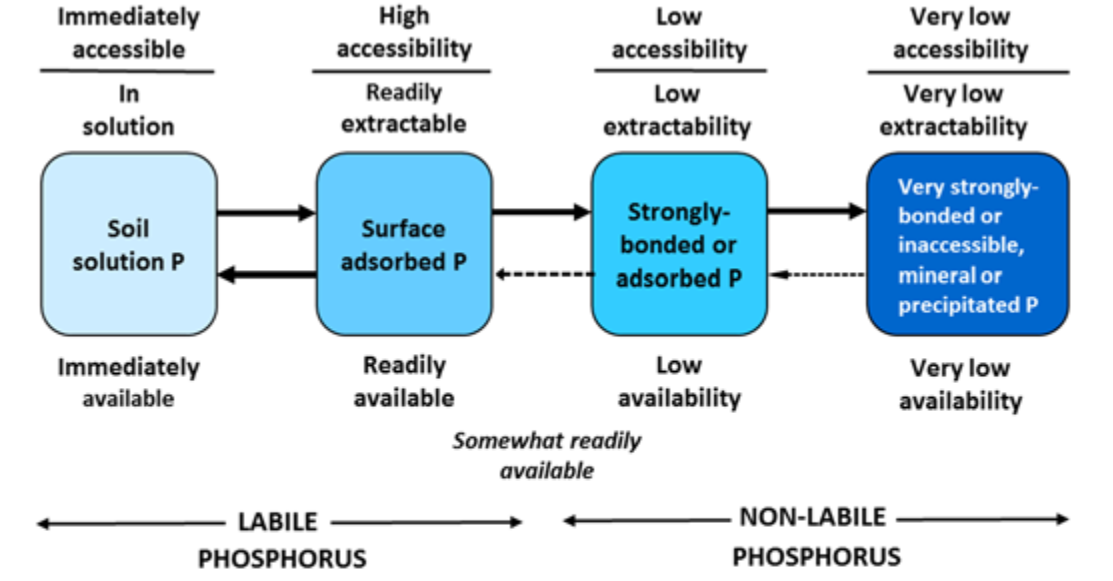
¶ Figure 1. Soil Phosphorus Fractions and Their Relative Availability for Plant Nutrition
¶ C. Availability of fertilizer phosphorus
- Availability and fixation affected by quantity and intensity factors
- Intensity factor
- Is phosphorus concentration in soil solution only
- Is phosphorus that is immediately available for uptake and assimilation (see Fig. 1)
- Is subject to organic assimilation, surface adsorption, and mineral precipitation reactions
- Quantity factor
- Includes various organic phosphorus, adsorbed phosphate, and mineral phosphorus fractions
- Includes both labile and non-labile fractions
- Availability of different fractions for uptake and assimilation during growing season is variable, ranging from readily available to very low
- Quantity is relative amount of these fractions
- Intensity factor
- Buffering capacity and phosphorus fixation are related
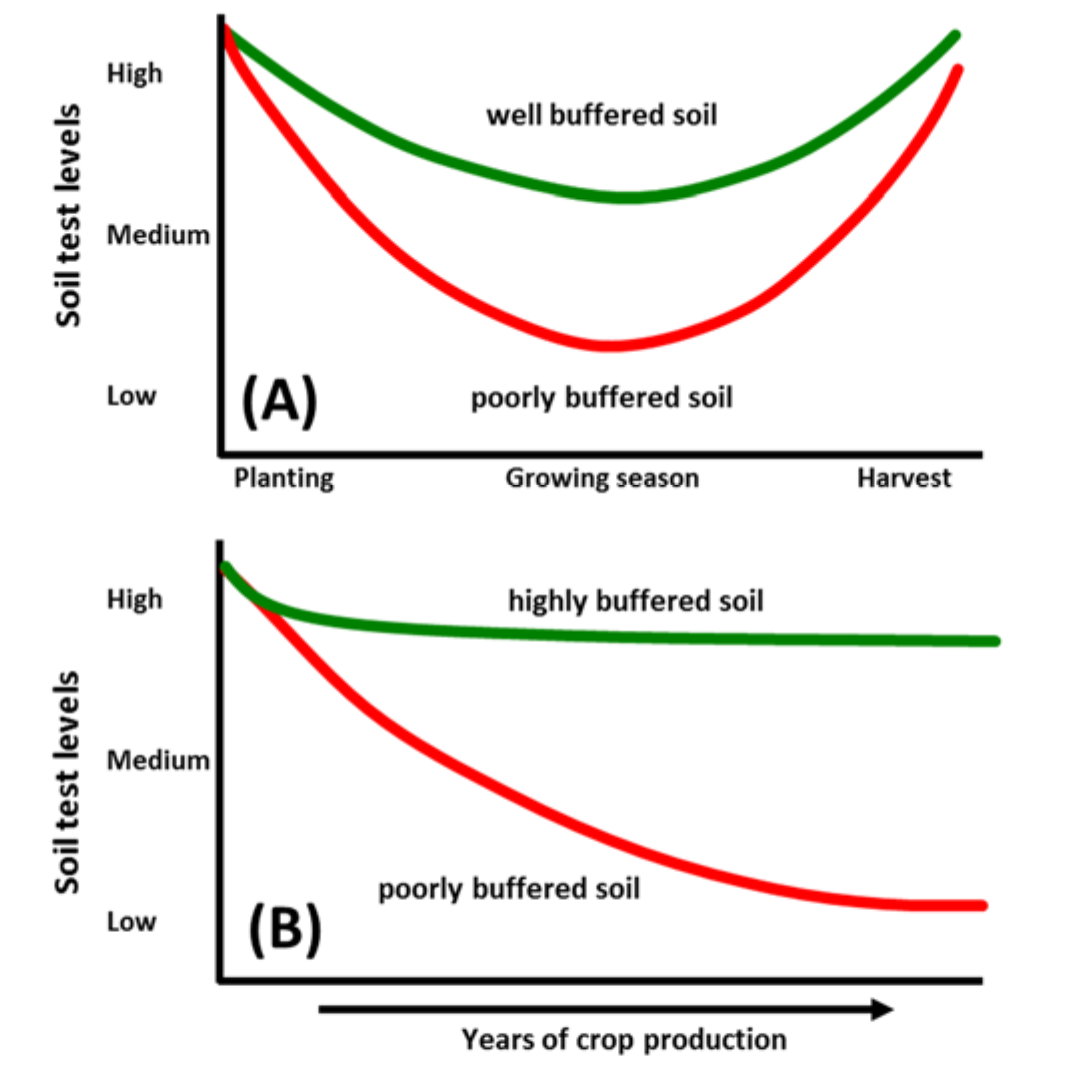
- Buffering capacity
- Ability of organic and mineral soil solids (quantity) to maintain phosphorus concentrations in soil solution (intensity)
- Capacity of soil phosphorus reserves to replenish and maintain phosphorus taken up by plant roots from solution
- Poorly buffered soil: Phosphorus reserves unable to sustain required concentration in solution phosphorus
- Quantity unable to maintain intensity
- Well buffered soil: Phosphorus reserves able to meet and/or exceed required concentration in solution
- Quantity able to maintain intensity
- Buffering capacity
- Relative phosphorus “availability”
- Adding fertilizer phosphorus
- Very soluble, increases solution phosphorus concentrations
- Increases phosphorus “intensity” in short term
- Phosphorus not removed by plant uptake increases “quantity” in longer term
- Phosphorus fixation reduces intensity
- Fixation initially converts water soluble phosphorus converted to soluble mineral and organic forms, then gradually into less soluble forms
- Does provide reservoir of phosphorus compounds to replenish solution phosphorus
- Phosphorus buffering capacity can be increased by adding fertilizer phosphorus
- Labile phosphorus
- Forms that rapidly replenish solution phosphorus
- Portion of adsorbed phosphorus that dissociates easily
- Includes some organic phosphorus from rapidly decomposable organic matter fractions
- Non-labile phosphorus
- Slowly replenishes soil solution phosphorus or labile phosphorus forms
- Strongly adsorbed phosphorus
- Phosphorus in stable, slowly, and very slowly degradable organic materials
- Mineral phosphates with limited or no solubility
- Adding fertilizer phosphorus
¶ Figure 2. Relative Comparisons of Soil Buffering Capacity in (A) Short-Term and (B) Long-Term
¶ D. Phosphorus fixation
- Various mechanisms remove inorganic phosphorus from soil solution
- Phosphorus that is mineralized from organic materials, applied as soluble fertilizers, or solubilized from other sources is subject to variety of inorganic physical/chemical reactions
- Precipitation/dissolution
- Formation or destruction of secondary phosphorus minerals (precipitates) in clay-sized particles (< 2 µm diameter)
- Some precipitates have defined, crystalline structure
- Some precipitates have amorphous structure
- Are “structurally disordered”; not organized in definite lattice pattern
- Adsorption/desorption
- Phosphorus is retained and released from mineral surfaces
- Phosphorus fixation is continuum of reactions
- No clear boundary between adsorption and amorphous precipitates
- Type of fixation varies with soil conditions
- Soil solution pH
- pH affects concentration of soluble cations that may combine with phosphate ions
- Affects characteristics of mineral surfaces
- Acid soils may fix two times more phosphorus per unit of surface area than comparable neutral to calcareous soils
- Adsorbed phosphorus is held with five times more bonding energy in acid soils than in comparable calcareous soils
- Phosphate and cation concentrations
- Adsorption dominates at lower concentrations
- Precipitation dominates higher concentrations
- Soil solution pH
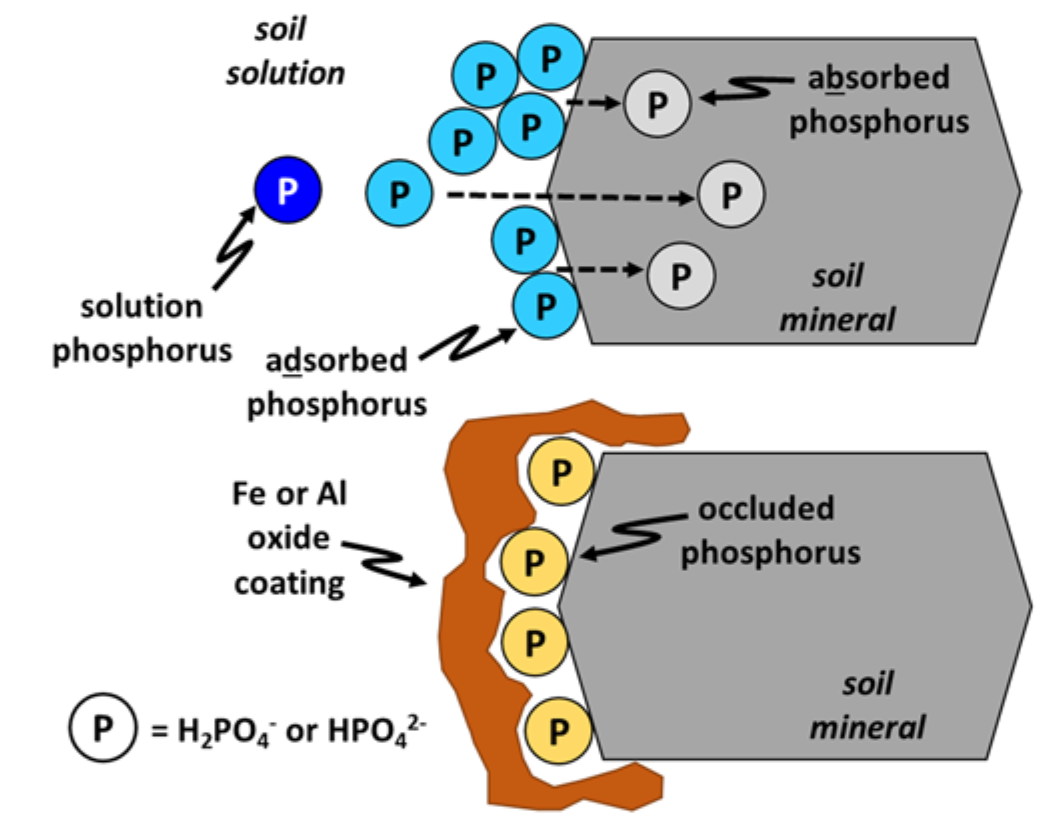
¶ Figure 3. Absorption of Adsorbed Phosphorus Into Soil Minerals and Subsequent Occlusion of Adsorbed Phosphorus
¶ E. Adsorption
- Process by which atoms, molecules, or ions are taken up from soil solution and retained on surfaces of solids by chemical or physical binding
- Fine-textured soils have larger adsorptive capacities than coarse-textured soils
- Higher clay content provides larger surface area
- Acidic soils have larger adsorptive capacities than neutral to calcareous soils
- Aluminum oxides and iron oxides have larger adsorptive capacities than carbonates
- Amorphous oxides have larger adsorptive capacities than crystalline forms
- Amorphous structures are not organized in definite lattice pattern; have larger surface areas
- Can occur as discrete particles or as coatings and films on other soil particles
- Adsorbed phosphorus may become trapped by oxide coating on mineral surface; called “occluded” phosphorus (see Fig. 3)
- Adsorbed phosphate ions may diffuse into solid minerals and become “absorbed” into mineral structure (see Fig. 3)
- Higher fertilizer rates are required to maintain adequate soil solution phosphorus in soils with high retention capacity (high capacity to adsorb and absorb)
- Some soils can fix large amounts of phosphorus, keeping soluble phosphorus low, but this high retention capacity can be exceeded
- Example: Continued applications of very high manure rates can overcome retention capacity
- Fine-textured soils have larger adsorptive capacities than coarse-textured soils
- Adsorption in acid soils
- Most phosphorus in H2PO4- form
- Aluminum and iron oxides/hydroxides are main mineral surfaces
- Surfaces have net positive charge
- Adsorption readily occurs on edges of broken clay minerals
- Characteristics of oxide, hydroxide mineral surfaces
- Have net positive charge in acid conditions
- Positive charge attracts anions; phosphate and others
- Phosphate ions displace –OH- and -OH2- groups
- Bonds to aluminum and iron oxide surfaces
- Specific adsorption: simple exchange reaction
- Bridging complexes can form between phosphate ions and metal oxide surfaces
- e.g., Al-O-Phosphate
- More stable; stronger bonds than simple adsorption
- Desorption more difficult for complexes
- Have net positive charge in acid conditions
- Labile phosphorus
- Phosphate bonded through one Al-O-P bond
- Readily desorbed from surface to replenish soil solution
- Also referred to as "active" phosphorus
- Non-labile phosphorus
- Phosphate bonded through two Al-O-P or Fe-O-P bonds
- Phosphate not easily desorbed from mineral surface to soil solution
- Adsorption to clay “edges”
- Broken edges of clay minerals expose –OH- groups
- Similar to –OH- exchange on aluminum oxide and iron oxide surfaces
- This type of adsorption greater by 1:1 clays (e.g., kaolinite) than by 2:1 clays (e.g., monmorillonite)
- Adsorption in calcareous soils
- Carbonates are main mineral surfaces in alkaline conditions
- Stable carbonates form at about pH 7.8 and higher in calcareous soils
- Small quantities of HPO42- may replace CO32- in calcium carbonate surfaces
- Some adsorption on Al(OH)3 and Fe(OH)3 mineral surfaces
- Carbonates are main mineral surfaces in alkaline conditions
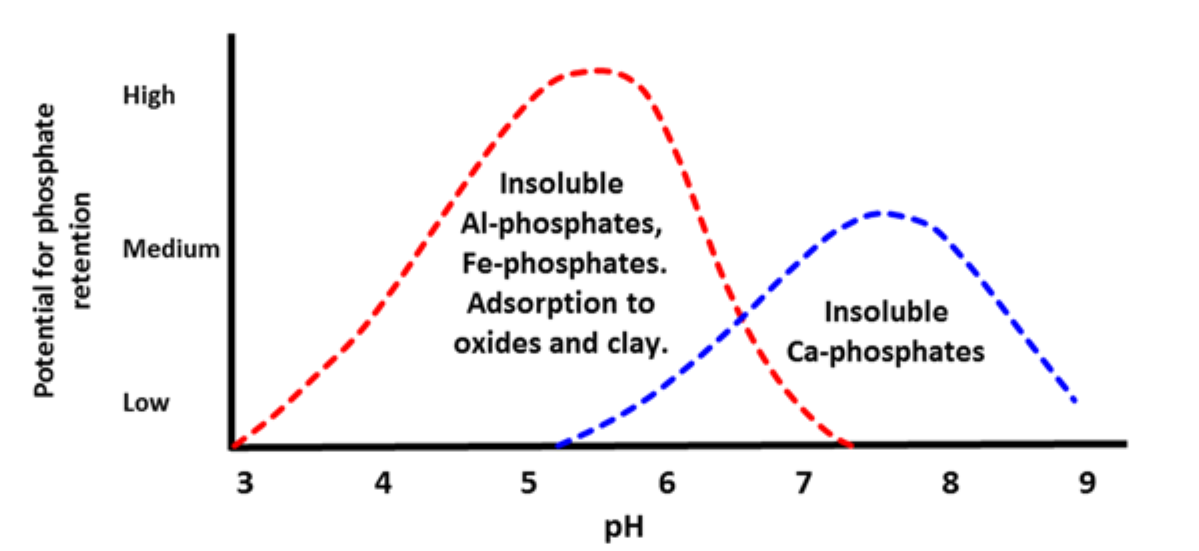
¶ Figure 4. Soil pH Effect on Phosphorus Adsorption and Mineral Precipitation
¶ F. Precipitation
- Process of forming solid compound(s) from ions or compounds that have been in solution
- Solution pH and solubility of various Al-, Fe-, and Ca-phosphates ultimately controls concentration of soil solution phosphorus
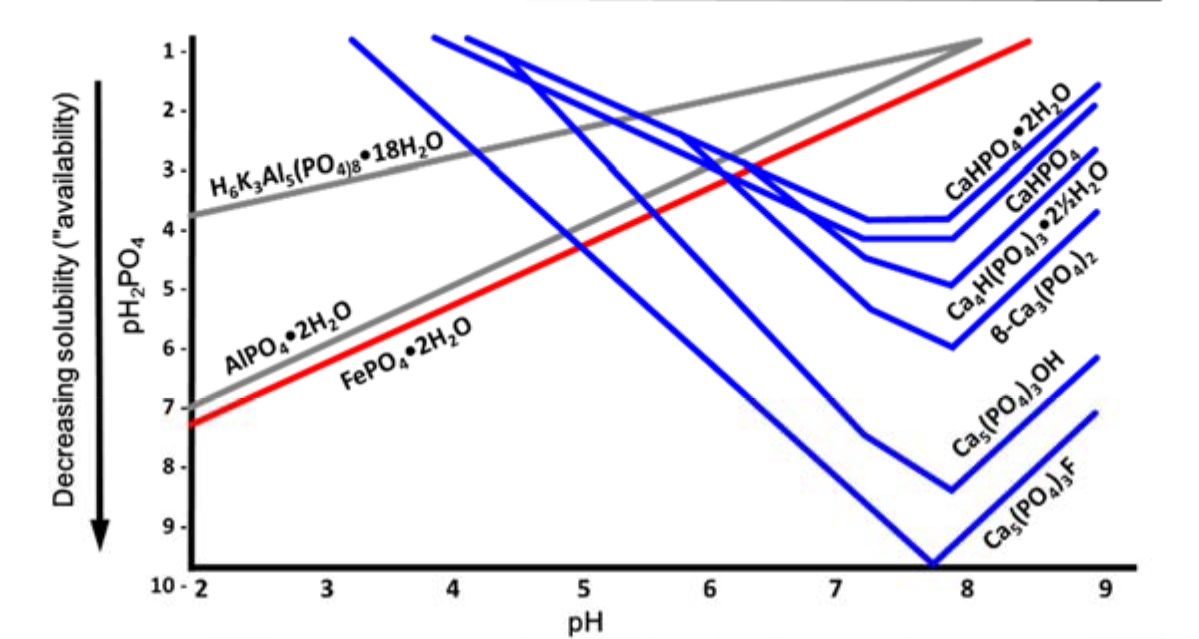
- “Optimum” phosphorus availability within soil pH range of 6 to 7 (see Fig. 4)
- pH range of 6 to 7 is between most insoluble zones of aluminum/iron phosphates and of calcium phosphates
- Precipitation reactions can be very slow
- i. Initial mineral precipitates are generally quite soluble; may be part of labile fractions (i.e., “readily” and “somewhat readily” available)
- ii. May form more stable, less soluble minerals over time; become part of non-labile fractions
- Precipitation in acid soils
- Aluminum and iron are main soluble cations
- Leads to precipitation of aluminum phosphate and iron phosphate minerals (see Table1 and Fig. 5)
- Example: FePO4•2H2O + H2O ↔ H2PO4- + H+ + Fe(OH)3
- If acidity (H+) increases, equilibrium moves reaction to left
- Iron phosphate precipitates and solution phosphorus decreases
- If acidity (H+) decreases, equilibrium moves reaction to right
- Iron phosphate dissolves and solution phosphorus increases
- When plant roots take up H2PO4-, equilibrium also moves reaction to right
- Iron phosphate dissolves to resupply soil solution phosphorus
- Solid iron phosphate will maintain H2PO4- at an equilibrium concentration level
- Equilibrium concentration may be very low, depending on pH
- If acidity (H+) increases, equilibrium moves reaction to left
- Aluminum and iron are main soluble cations
- Precipitation in neutral and calcareous soils
- Calcium is main soluble cation
- Leads to precipitation of calcium phosphate minerals (see Table 1 and Fig. 5)
- Example: CaHPO4•2H2O + H+ ↔ Ca2+ + H2PO4- + 2H2O
- If acidity (H+) decreases, equilibrium moves reaction to left
- Calcium phosphate precipitates and solution phosphorus decreases
- If acidity (H+) increases, equilibrium moves reaction to right
- Calcium phosphate dissolves and solution phosphorus increases
- If acidity (H+) decreases, equilibrium moves reaction to left
- When plant roots take up H2PO4-, equilibrium also moves reaction to right
- Calcium phosphate dissolves to resupply soil solution phosphorus
- Solid calcium phosphate will maintain H2PO4- at an equilibrium concentration level
- Equilibrium concentration may be very low, depending on pH
- Calcium is main soluble cation
¶ Table 1. Common soil phosphorus minerals |
|
|---|---|
| Acid Soils | |
| Variscite | AlPO4*2H2O |
| Strengite | FePO4*2H2O |
| Tarakanite | H6K3Al5(PO4)8*18H2O |
| Neutral and Calcareous Soils | |
| Dicalcium phosphate dehydrate (DCPD) | CaHPO4•2H2O |
| Dicalcium phosphate (DCP) | Ca5HPO4 |
| Octacalcium phosphate (OCP) | Ca4H(PO4)3•2½H2O |
| β‐ttricalcium phosphate (βTCP) | β‐Ca3(PO4)2 |
| Hydroxyapatite (HOA) | Ca5(PO4)3OH |
| Fluoroapatite (FAP) | Ca5(PO4)3F |
¶ Figure 5. Solubilities of Common Soil Phosphorus Minerals Change With pH