⇦ Back to Soil Fertility and Plant Nutrition Home
¶ Introduction
Nitrogen is plentiful in the environment, about 78% of the air we breathe. It undergoes many different transformations in that same environment, changing from one form to another. Some forms are available for crop uptake; some are not.
Table 1 shows some typical season-end outcomes of fertilizer nitrogen applied to corn. This Crop File provides general information about nitrogen transformations important for agronomic and horticultural crop management.
¶ A. Mineralization
- Release of organic nitrogen as plant available ammonium-nitrogen (NH4-N)
- Carried out by diverse groups of heterotrophic bacteria and fungi
- Heterotrophs: organisms that require complex organic nitrogen and carbon materials as food source
- Bacteria dominate fungi in neutral to alkaline soils
- Fungi dominate in acidic soils
- Soil organic matter is about 5% nitrogen
- About 1% to 4% of this organic nitrogen is mineralized each year
- Requires warm temperatures, adequate moisture, good oxygen supply (aeration)
- Carried out by diverse groups of heterotrophic bacteria and fungi
- Aminization
- Cleavage of complex protein molecules into smaller, amine-containing subunits
- Amine = R-NH2
- R group: abbreviation for any group in which a carbon or hydrogen atom is attached to the rest of the molecule
- Proteins + H2O → amino acids + amines + urea + CO2 + energy
- Multiple step-wise process
- End-products of activities of one group of microbes provides food for next group until decomposition is finished
- Cleavage of complex protein molecules into smaller, amine-containing subunits
- Ammonification
- Final step; ammonium may be taken up by plants or transformed
- R-NH2 + H2O → NH3 + R-OH + energy
- NH3 + H2O → NH4+ + OH-
- Final step; ammonium may be taken up by plants or transformed
- Affected by soil temperature and moisture
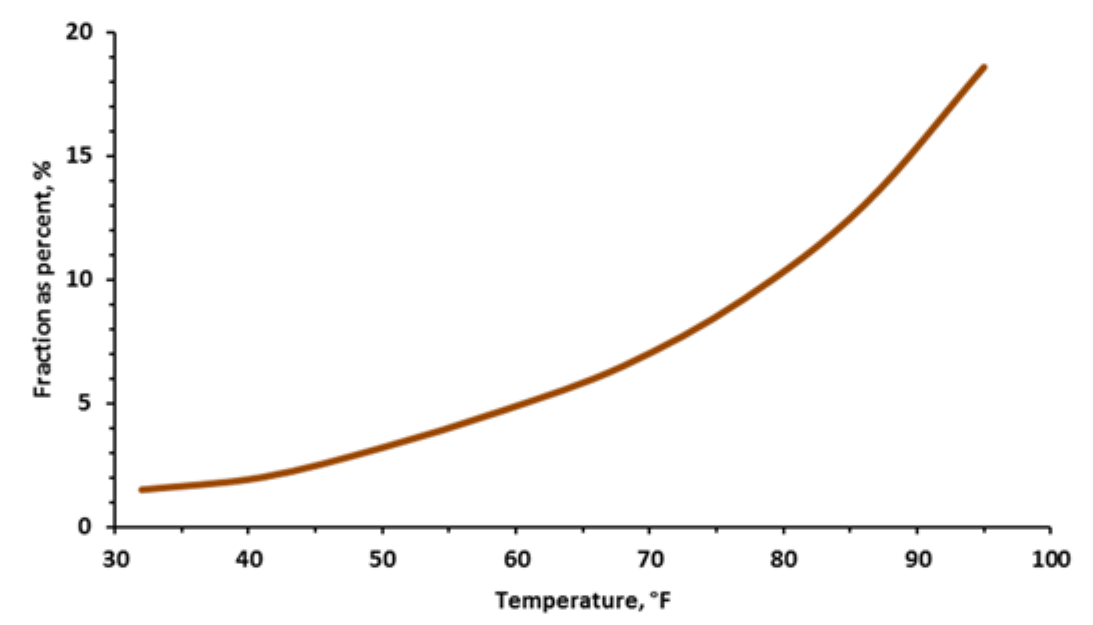
- Optimal temperature range for mineralization about 85° to 95°F or 30° to 35°C (see Fig. 1)
- Mineralization rate doubles for each 18°F (10°C) increase between soil temperatures from 40° to 105°F (5° to 40°C)
- Mineralization rates decline fairly quickly when temperature is below or is above this range
¶ Table 1. Generalized Fate of Fertilizer Nitrogen Applied to Corn* |
||
|---|---|---|
| Soil Texture | ||
| Coarse | Medium/Fine | |
| Fate of applied N: | --- % of Applied Nitrogen --- | |
| Plant uptake (first year) | 40 – 60 | 50 – 60 |
| Remains in soil as organic/inorganic N | 20 – 25 | 25 – 30 |
| Lost from root zone:: | ||
| Denitrification | 5 – 10 | 15 – 25 |
| Leaching | 15 – 20 | 0 – 10 |
| * Average values over years for soils in the Corn Belt and southeastern U.S. and irrigated soils of the Great Plains and western valleys | ||
¶ B. Immobilization
- Reverse of mineralization
- Uptake of inorganic or mineral nitrogen from the soil and incorporation into organic nitrogen forms by microbes
- Often temporary condition; occurs during decomposition activities if nitrogen is limiting
- “Normal” mineralization resumes after decomposition is completed
- Balance between mineralization and immobilization determined by carbon-to-nitrogen (C:N) ratio of organic matter
- Stable soil organic matter has C:N ratio of about 10:1, ranging from 9:1 to 12:1
- C:N ratio = X parts carbon for each 1 part nitrogen
- C:N ratio for microbial nutrition equivalent to carbohydrate-to-protein ratio for livestock nutrition
- Stable soil organic matter has C:N ratio of about 10:1, ranging from 9:1 to 12:1
- Microbial populations increase rapidly when residues (i.e., carbon source) are incorporated into soil
- Microbes require nitrogen and other nutrients to assimilate readily decomposable carbon compounds
- Decomposition activity continues until carbon source is exhausted
- Population declines as microbes “starve”
- Decomposition of microbial bodies releases mineral nitrogen back into the soil (i.e., mineralization)
- Supplemental nitrogen from mineral sources may be needed during decomposition
- Microbes need nitrogen in C:N ratio of about 8:1
- Crop residues have C:N ratios ranging from 20:1 to 80:1
- Microbes compete with plants for NH4+ and NO3-
- Are “first to the table”, before plants
- If soil NH4+ and NO3- supplies are low, plants can become nitrogen deficient during decomposition process
- Immobilization of mineral nitrogen occurs when residues have C:N ratio greater than about 30:1
- Mineralization at “normal” rates if residue nitrogen content is 1.2% to 1.5% on dry matter basis
- Equivalent: 7.5% to 9.5% crude protein content
- Mineralization at “normal” rates if residue nitrogen content is 1.2% to 1.5% on dry matter basis
- Condition is temporary, but may last for days or weeks
¶ C. Nitrification
- Conversion (biological oxidation) of NH4+ to NO3-
- Source of NH4+ can be OM or fertilizer
- Oxidation state of nitrogen increases from -3 to +5
- Conversion from immobile NH4+ ion to mobile NO3- ion increases potential for nitrogen leaching
- Two-step process
- Primarily performed by two types of autotrophic, aerobic bacteria, but also some other species
- Obtain energy from nitrogen oxidation
- Obtain carbon from CO2
- Nitrosomonas bacteria
- Step 1: 2NH4+ + 3O2 → 2NO2- (nitrite) + 2H2O + 4H+
- Nitrobacter bacteria
- Step 2: 2NO2- + O2 → 2NO3-
- Nitrite (NO2-) is toxic
- Generally does not accumulate in soil
- Conversion rate of nitrite to nitrate much faster than conversion rate of ammonium to nitrite
- Primarily performed by two types of autotrophic, aerobic bacteria, but also some other species
- Gradually acidifies soil
- Accounts for acidifying effect of anhydrous ammonia, urea, and ammonium-based fertilizers
- Two moles of H+ produced per mole of NH4+ converted to nitrate
- Each pound of nitrogen requires equivalent of two to three pounds of agricultural lime to neutralize acidity produced during nitrification
- Accounts for acidifying effect of anhydrous ammonia, urea, and ammonium-based fertilizers
- Soil conditions affect speed of nitrification
- Most rapid with warm temperatures and moist soil
- Optimal temperature range for nitrification about 75° to 95°F (24° to 35°C)
- Bacteria require well-aerated soil
- Free oxygen (O2) is reactant in both steps
- Bacteria are “obligate aerobes”
- Cannot make ATP and grow in the absence of oxygen
- Cannot reproduce and will die in absence of oxygen
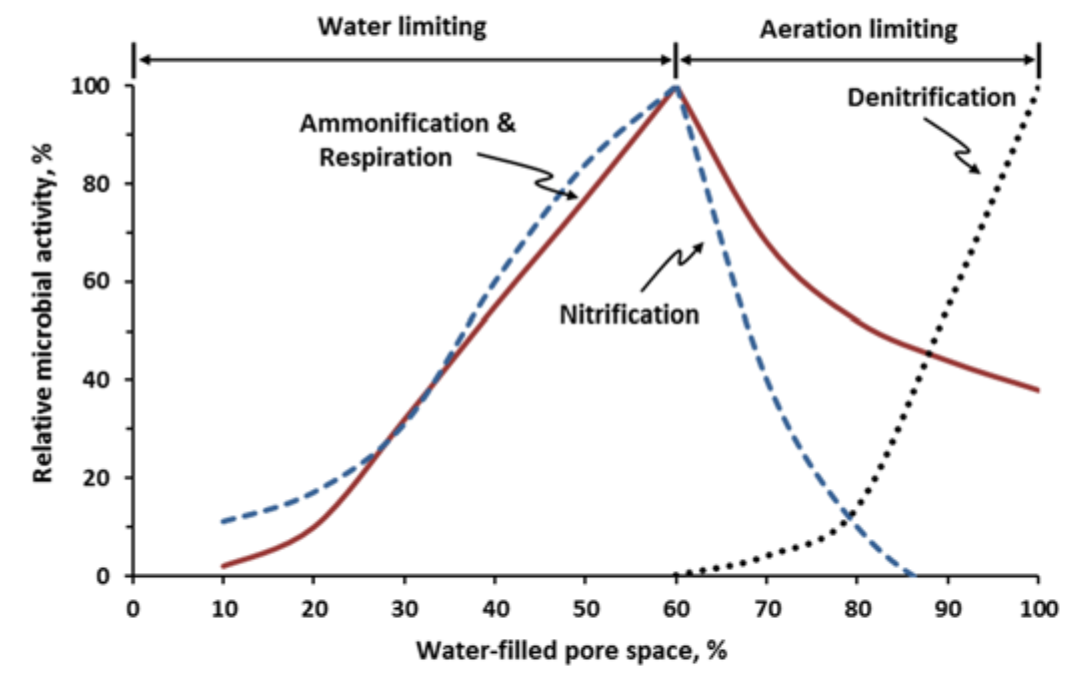
- Optimal soil moisture when 60% of soil pore spaces filled with water, 40% with air (see Fig. 2)
- Most rapid at higher pH, with optimum about pH 8.5
- Equilibrium of reaction also favors high pH
- Bacteria need adequate calcium and phosphorus
¶ Figure 1. Fraction of Organic Nitrogen
¶ D. Denitrification
- Gaseous losses of nitrogen from nitrate
- Conversion of nitrate (NO3-) in soil solution to gaseous nitrogen (N2) and nitrous oxide (N2O)
- Increased problem in higher rainfall areas, in no-till systems, and under irrigation
- Losses of 5% to 20% of nitrate-nitrogen are not uncommon; can reach 50% loss in severe cases
- Significant problem in paddy rice production
- Process can vary widely across field due to variable soil conditions
- Performed by anaerobic bacteria
- Pseudomonas, Bacillus, and other species are “facultative anaerobes”
- Are able to grow either with or without free oxygen
- Capable of switching to fermentation or anaerobic respiration if oxygen is absent
- Can use oxidized nitrogen (NO3) as their O2 source for respiration when oxygen is absent
- Requires presence of nitrate
- Does not affect ammonium-nitrogen or organic nitrogen until converted to nitrate
- Requires absence of oxygen (anaerobic conditions)
- Waterlogged and saturated soils
- When 80% to 100% of soil pores filled with water, less than 20% of pores filled with air (see Fig.2)
- Recurring problem in poorly drained soils
- Localized anaerobic zones
- Low oxygen in soil microsites, e.g. around roots or decomposing residues
- Oxygen replaced by carbon dioxide from microbial respiration
- Slower oxygen diffusion due to many micropores in fine-textured soils or to pore spaces that were lost due to soil compaction
- Waterlogged and saturated soils
- Denitrifying bacteria require organic matter
- Readily decomposable organic materials are an energy source
- e.g., 5(CH2O) + 4NO3- + 4H+ → 5CO2 + 2N2O↑ + 7H2O, where CH2O represents carbohydrate unit
- Edible oils, molasses, ethanol/methanol have been used as soluble carbon sources to stimulate denitrification in high nitrate ground water
- Readily decomposable organic materials are an energy source
- Conditions favorable for denitrification loss
- Large supply of nitrate
- Nitrogen in ammonium or other forms is not susceptible to denitrification loss until nitrified
- Anaerobic conditions
- Increased problem in wet, poorly drained, and compacted soils
- Increases rapidly when 80% or more of pore spaces are filled with water
- Loss becomes significant if soil is saturated for 36 hours or more
- Losses increase with increasing length of time that soil is saturated or waterlogged increases
- Warm temperatures
- Rapid increase of microbial activity between 50° and 80°F
- Activity most rapid between 80° to 100°F
- Increased nitrification rate increases potential supply of nitrate
- Elevated organic matter
- Greater losses in high organic matter soils
- Larger reservoir of soluble carbon
- Higher water holding capacity
- Waterlogging stimulates release of soluble carbon
- Applied manure can double denitrification rates
- Greater losses in high organic matter soils
- Soil pH over 5.5
- Denitrification negligible if pH is below 5.0
- Growing plants?
- May encourage denitrification
- Root exudates supply soluble carbon
- Root respiration consumes oxygen; lower O2
- May limit denitrification
- Deplete nitrate supply through uptake
- Removes soil water from pores, increases percentage of air-filled pore spaces
- May encourage denitrification
- Large supply of nitrate
- Greater problem in no-till systems
- Retaining residues provides source of soluble carbon
- Compared to conventional tillage, have higher proportion of micropores
- Macropores promote soil drainage
- Micropores store water
- Residue cover conserves soil moisture; increases number of water filled pore spaces
¶ Figure 2. Relative Biological Activity as Affected by Percent of Soil Pores Filled With Water
¶ E. Volatilization
- Gaseous loss of nitrogen as ammonia (NH3)
- Losses can occur with ammonia, ammonium-based, or urea-based fertilizers
- Plants can take up ammonium, but not urea or ammonia
- General reaction: NH4+ → H+ + NH3↑
- Ammonia is volatile gas
- Can be lost to atmosphere
- Water solution may contain both ammonium (NH4-N) and ammonia (NH3-N) nitrogen forms
- Below pH 7.0, over 99% NH4+
- At pH 8.0, about 95% NH4+, 5% NH3
- At pH 9.0, about 64% NH4+, 36% NH3
- Losses can occur with ammonia, ammonium-based, or urea-based fertilizers
- Ammonia loss favored by high pH
- Ammonium (NH4+) is ionized in water solution
- Can adsorb to exchanges sites on soil particles
- Ammonia (NH3) is non-ionic in water solution
- Remains in soil solution
- More ammonia is in soil solution at higher pH
- Helps pull reaction to right; toward NH3
- NH4+ → H+ + NH3↑
- Ammonium (NH4+) is ionized in water solution
- Acid to neutral soils
- Losses greater from urea-based than ammonium-based fertilizers
- Initial reaction of ammonium is acidic
- 2NH4+ + 3O2 → 2NO2- + 2H2O + 4H+
- Urea hydrolysis increases pH around granule
- CO(NH2)2 + H+ + 2H2O → 2NH4+ + HCO3-
- Consumes H+ and increases pH
- pH can be well above 7
- Leads to: NH4+ + HCO3- → NH3↑ + H2O + CO2
- Losses greater from urea-based than ammonium-based fertilizers
- Calcareous soils
- Urea losses still potentially very high
- Ammonium fertilizers more subject to volatilization than in acid to neutral conditions
- React with carbonates
- Ultimate reaction = NH4+ + HCO3- → NH3↑ + H2O + CO2
- Ammonia losses greater from ammonium phosphates and sulfates than from soluble ammonium salts (chlorides and nitrates)
- Released ammonia can be toxic to seedlings with seed-placed applications
- Conditions favorable for volatilization losses
- Alkaline pH, calcareous soil
- Surface-applied nitrogen fertilizers without soil incorporation
- Includes surface-applied manure
- “Drying” conditions
- Warm, breezy weather with moist soil surface
- Evaporation creates high humidity zone on soil surface and around fertilizer prill/droplet
- Exposed portion of prill/droplet begins to dissolve, but net movement of water is upward so fertilizer is not incorporated into soil
- Ammonia remains in solution on soil or residue surface, available for volatilization loss
- Also occurs with urea fertilizer applied to light snow cover followed by sunny weather
- Heavy residue cover or thatch
- Limits or prevents fertilizer-to-soil contact
- Liquid vs. solid fertilizer materials
- Dissolving of dry fertilizer prill creates mini-zone of highly concentrated ammonium or urea around prill
- Droplets from broadcast liquid fertilizer materials are more diffuse at same nitrogen rate
¶ F. Nitrogen fixation
- Atmospheric nitrogen (N2) must be transformed (fixed) into plant-available forms
- Nitrogen is often limiting, even though air is 78% N
- 70 million pounds of nitrogen above every acre
- Industrial nitrogen fixation
- Haber-Bosch process
- N2 + 3H2 → 2NH3
- NH3 (anhydrous ammonia) used directly as fertilizer
- Used to produce other forms of N fertilizer
- N2 reduced with high energy input
- Requires high temperature (1,200°C) and high pressure (500 atmospheres)
- Requires metal catalyst
- Haber-Bosch process
- Biological nitrogen fixation
- Variety of microorganisms can fix N2
- Are both symbiotic and free-living types
- Rhizobia and legumes
- Most important agricultural relationship
- Symbiotic Rhizobia bacteria species form nodules (abnormal swelling) on roots
- Host plant receives fixed nitrogen from bacteria
- Bacteria receive photosynthate from host plant
- Variety of microorganisms can fix N2
- Rhizobia and legume symbiosis
- Are specific relationships
- Different legume groups require different Rhizobia species
- Seed inoculation
- Necessary for first time planting of new legume
- Introduces improved strain of Rhizobia to soil
- Strains within Rhizobia species differ in potential to fix atmospheric nitrogen
- Must match crop varieties with improved strains
- Amounts of nitrogen fixed will vary
- Perennial legumes: about 100 to 200 lb./ac/yr
- Annual legumes: about 40 to 80 lb./ac/yr
- Nitrogen available to succeeding crop depends upon amount of nitrogen removed in harvested legume
- Are specific relationships
- Factors affecting nitrogen fixation
- Soil pH
- Low pH harmful to Rhizobia and legume roots
- Aluminum, manganese toxicity
- Calcium, phosphorus, molybdenum deficiency
- Species and strains differ in sensitivity
- R. meliloti (alfalfa, sweet clover) most sensitive to low pH
- Some strains more tolerant to low pH
- Low pH harmful to Rhizobia and legume roots
- Soil nitrogen availability
- High soil nitrate, reduced nitrogenase activity, less nitrogen fixation
- Nitrate taken up by mass flow during water uptake; passive process
- Metabolic reduction of NH4+ to NO3- competes for photosynthate
- Crop growth and management
- Conditions that promote high photosynthetic rates and growth increase nitrogen fixation
- Any factor that reduces stand or yield reduces nitrogen fixation
- Cutting frequency and timing
- Presence/absence of stress
- Soil pH
- Other nitrogen fixation relationships
- Azolla--Anabaena
- Water fern (Azolla) and blue-green algae/cyanobacteria (Anabaena)
- Fixes significant amounts of nitrogen in rice paddies
- Most other nitrogen fixation relationships are of limited importance to agriculture
- Are very important to natural ecosystems and agroforestry
- Azolla--Anabaena