⇦ Back to Soil Fertility and Plant Nutrition Home
¶ Introduction
¶ A. Nitrogen in the plant
- Primary macronutrient, required by plants in large amounts
- Typically is most limiting nutrient in unfertilized systems
- Total leaf tissue content typically ranges from 1.0% to 6.0% N (dry weight)
- Forms and functions
- Component of amino acids, amides, amines
- Are building blocks and intermediary compounds
- Component of proteins, chlorophyll, nucleic acids
- Proteins/enzymes regulate biochemical reactions
- Nitrogenous bases of DNA, RNA
- Component of amino acids, amides, amines
- Mobile in plant
- Most nitrogen taken up by roots as inorganic forms
- Translocated from older leaves to younger leaves
- Nitrogen uptake
- Most nitrogen taken up as nitrate ion (NO3-) or ammonium ion (NH4+)
- Most crops grow best with a combination of nitrate and ammonium
- Plants adapted to acid soils generally grow best with NH4+
- Solanaceae species (i.e., nightshade family) grow best with high NO3-
- Direct absorption of NH3 by leaves
- Amounts depend upon air concentration
- Can also volatilize through leaves
- Nitrate vs. ammonium
- Relative amounts absorbed by roots depend upon soil conditions
- NO3- ion generally most prevalent in warm, moist, well aerated soils
- NH4+ ion uptake favored by neutral pH; NO3- uptake favored by low pH
- Nitrate uptake
- Nitrate must be reduced (NO3- → NH3) before amino acids, etc., are synthesized
- Nitrate concentrates in petioles and in base or lower portions of main stems
- Plants can tolerate excess nitrate better than excess ammonium
- Excess NO3- can be safely stored in vacuoles at comparatively high levels
- Affects cation/anion balance
- Nitrate uptake decreases anion uptake: phosphate (HPO42-, H2PO4), sulfate (SO42-), chloride (Cl-)
- Nitrate uptake increases cation uptake: potassium (K+), calcium (Ca2+), magnesium (Mg2+),
- Roots release HCO3- (OH-) during NO3- uptake; increases rhizosphere pH
- Ammonium uptake
- Does not have to be reduced to other forms
- Conserves energy
- Higher protein, carbohydrates in plants
- Affects cation/anion balance
- Nitrate uptake reduces cation uptake: calcium (Ca2+), magnesium (Mg2+), potassium (K+)
- Nitrate uptake increases anion uptake: phosphate (HPO42-, H2PO4), sulfate (SO42-), chloride (Cl-)
- Rhizosphere pH
- Roots release H+; acidifies rhizosphere
- Lower pH may help increase phosphate or iron availability
- Does not have to be reduced to other forms
- Deficiency symptoms
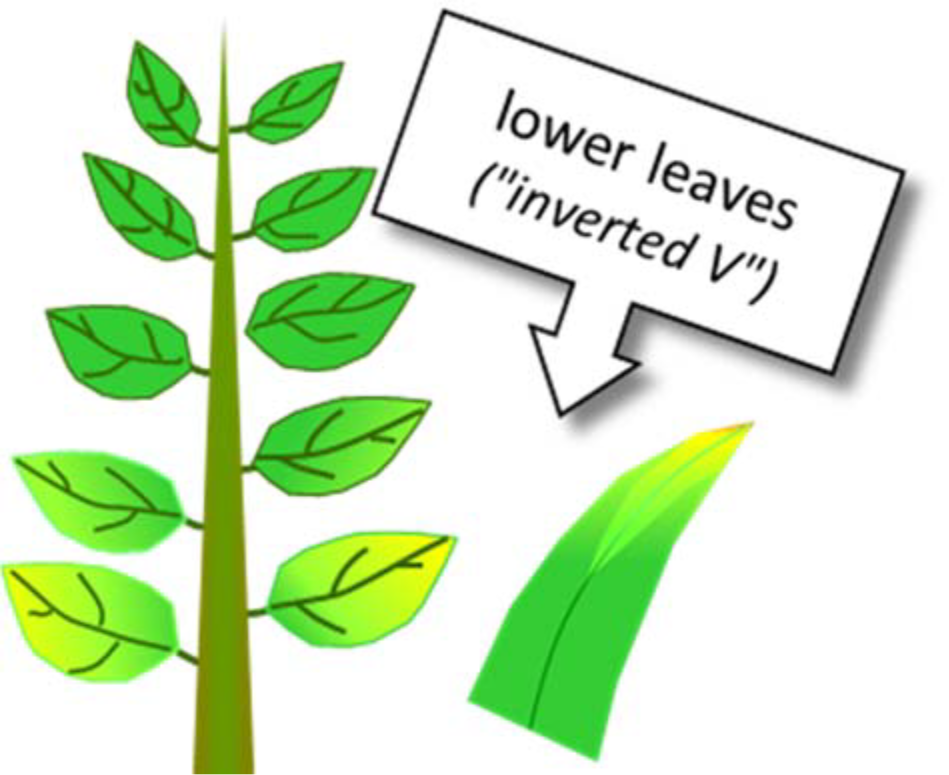
- Mobile within plant, so deficiencies occur first on lower, older leaves
- General chlorosis (yellowing) of leaves, followed by necrosis (tissue death)
- “Inverted V” is common symptom in grasses
- Chlorosis begins at midrib, about half-way to third of leaf length from tip
- Chlorosis extends diagonally toward leaf margins
- “Inverted V” is common symptom in grasses
- Nitrogen is integral part of chlorophyll structure
- Accounts for pale green/yellow color of nitrogen deficient plants
- Excess nitrogen
- Nitrogen stimulates vegetative growth
- Encourages high rate of photosynthesis
- Encourages carbohydrate utilization
- Delayed maturity, plants physiologically younger
- More attractive to insects, diseases
- Encourages development of soft, succulent plant tissue
- Weaker stems, increased lodging
- Less resistant to disease attach
- High ammonium levels can be toxic if not incorporated into carbon containing compounds after absorption
- May occur if ammonium is dominant form of nitrogen
- Toxicity due to ammonia formed within plant, NH4+ → NH3
- Tolerance levels fairly narrow
- Environmental problems
- Nitrate in drinking water
- Nitrogen in aquatic systems
- Ammonia toxic to fish
- Nitrate contributes to eutrophication
¶ Figure 1.
¶ B. Nitrogen in the soil
- 95% or more of total nitrogen found in organic matter
- Total nitrogen ranges from less than 0.2% N (in subsoils) to more than 2.5% N (in peat soils)
- Total nitrogen in top foot of most cultivated soils ranges from 0.03% to 0.40% N
- Inorganic forms of nitrogen
- Nitrate, NO3-
- Highly mobile
- Very soluble
- Not retained by soil colloids
- Ammonium, NH4+
- Is an exchangeable cation
- Retained by soil colloids
- Mobile in sandy, low CEC soil
- Elemental nitrogen, N2
- Inert, atmospheric nitrogen
- Nitrous oxide, N2O
- Non-flammable gas
- Nitric oxide, NO
- Colorless gas
- Nitrate, NO3-
- Forms absorbed by plants
- NO3- mainly moves to plant roots by mass flow
- NH4+ not considered mobile, moves largely by diffusion
- Ammonium ion (NH4+) only moves about 1 to 4 mm, similar to potassium ion (K+)
- Limited uptake by mass flow
- Organic nitrogen, elemental nitrogen, nitrous oxide, and nitric oxide not plant available
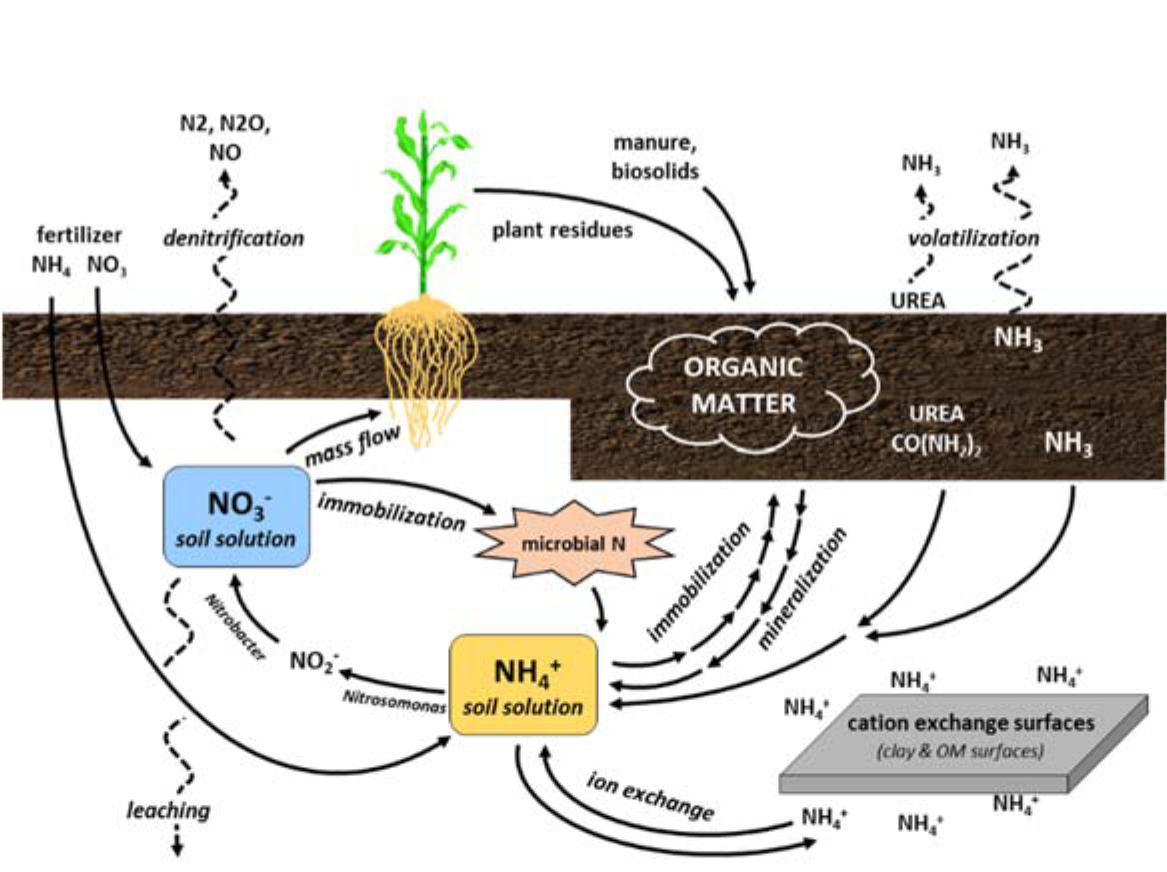
- Nitrogen sources
- Organic matter decomposition
- Plant residues
- Stable soil organic materials
- Biological fixation
- Symbiotic relationship between bacteria and plant (e.g., Rhizobia sp.)
- Non-symbiotic, free-living organisms
- Nitrogen fertilizers
- Applied manure, compost, biosolids
- Atmospheric deposition
- From lightning discharges
- From industrial activities (use of fossil fuels)
- Organic matter decomposition
- Soil transformations (for further information see other Crop Files)
- Mineralization: release of organic nitrogen as plant available ammonium-nitrogen (NH4-N)
- Immobilization: uptake of inorganic nitrogen from the soil and incorporation into organic nitrogen forms by microbes or plants
- Nitrification: conversion (biological oxidation) of ammonium (NH4+) to nitrate (NO3-)
- Denitrification: conversion of NO3- in soil solution to gaseous nitrogen (N2) and nitrous oxides (NxOx)
- Volatilization: gaseous losses of ammonia (NH3) from organic materials, ammonium-based fertilizers, or urea
- Nitrogen fixation: conversion of atmospheric nitrogen (N2) into plant-available forms
¶ Figure 2.
¶ C. Soil testing for nitrogen
- Inorganic nitrate-nitrogen, NO3-N
- Can be extracted with water or KCl solution
- Determined using colorimetry or ion selective electrode
- Based on analysis of soil sample or incremental samples collected from depth of effective root zone; depth may range from 1 to 4 feet
- Preplant nitrate test (PPNT)
- Nitrate-nitrogen found in soil analysis credited against crop nitrogen requirement
- Adapted to sub-humid and semi-arid areas; like Central Plains, western Intermountain, etc.
- Late-spring or pre-sidedress nitrate test (LSNT, PSNT)
- Index of nitrogen availability related to crop nitrogen response using test calibration data
- Adapted for humid regions, like Midwest, Corn Belt, Eastern U.S., etc.)
- Inorganic ammonium-nitrogen, NH4-N
- Exchangeable ammonium ion displaced by potassium ion (K+) in extracting solution
- Determined by colorimetry or ion selective electrode
- May be useful when urea-based or ammonium-based fertilizers have been recently applied
- Approaches to determine organic-nitrogen “availability”
- Chemical or biological methods
- Laboratory methods used to estimate amount of inorganic nitrogen that may be released from organic forms during growing season
- Not well calibrated to yield response
- Actual nitrogen released depends on field conditions, temperature, moisture, etc.
- Organic matter (total organic carbon)
- %OM multiplied by factor to estimate annual contribution (e.g., 10 or 20 lb N per % OM)
- Total nitrogen
- Multiply concentration by annual release factor
- e.g., 1% to 4% of total nitrogen per year
- Chemical extraction of biochemically active fraction
- Often well correlated with total organic carbon
- Permanganate oxidizable carbon (POXC)
- “Active organic matter”
- Amino-sugar nitrogen
- Several other methods
- Biological methods
- Incubate soil sample, followed by nitrate analysis
- Long-term method, incubation period is several days to several weeks
- Microbial respiration
- Measures carbon dioxide released by soil microbes
- Microbial activity generally related to nitrogen release
- Methods not well calibrated to field response
- Incubate soil sample, followed by nitrate analysis
- Chemical or biological methods
¶ D. Nitrogen nutrient sources
- Manure, compost
- Common analysis: 1.5% to 3% N (dry basis)
- Nitrogen content, availability depends on moisture, species, storage method, handling, and application
- Biosolids, compost
- Common analysis: 3% to 5% (dry basis)
- Final nitrogen content and availability depends on moisture, storage method, handling and application
- Organic materials
- Various materials, usually with high protein content
- e.g., alfalfa meal, cotton burr compost, fish meal, blood meal, etc.
- Highly variable nitrogen content
- Nitrogen release rate depends on C:N ratio, weather
- Various materials, usually with high protein content
- Anhydrous ammonia, NH3
- Common analysis: 82% N (82-0-0)
- High-pressure liquid produced by Haber-Bosch process
- Boiling point: -28°F (-33°C)
- Injected below soil surface
- Can be mixed with water to produce aqua ammonia (19% to 30% N)
- Used as commercial refrigerant
- base product of many industrial chemical production processes
- Urea, CO(NH2)2
- Common analysis: 45% to 46% N, (e.g., 45-0-0)
- Also known as “carbamide”
- Dry granular solid produced by reaction of ammonia and carbon dioxide
- Usually surface applied before or after seeding
- Common analysis: 45% to 46% N, (e.g., 45-0-0)
- UAN solution
- Common analysis: 28% to 32% N, (e.g., 28-0-0)
- One-half urea-nitrogen (14% to 16% urea-N)
- One-fourth ammonium-nitrogen (7% to 8% NH4-N)
- One-fourth nitrate-nitrogen (7% to 8% NO3-N)
- Clear to translucent liquid material
- Surface applied or soil injected before or after seeding
- Often applied through fertigation
- Common analysis: 28% to 32% N, (e.g., 28-0-0)
- Ammonium sulfate (AMS), (NH4)2SO4
- Common analysis: 21% N, 24% S
- Dry granular solid produced by reaction of ammonia and sulfuric acid
- Usually surface applied before or after seeding
- Water-soluble crystals used as herbicide additive
- Ammonium nitrate, NH4NO3
- Common analysis: 34% N, (e.g., 34-0-0)
- One-half ammonium-nitrogen (7% to 8% NH4-N)
- One-half nitrate-nitrogen (7% to 8% NO3-N)
- Dry granular solid produced by reaction of ammonia and nitric acid
- Readily attracts moisture in storage
- Cannot be stored in proximity to urea
- Usually surface applied before or after seeding
- ANFO (ammonium nitrate/fuel oil) is widely used industrial explosive
- Common analysis: 34% N, (e.g., 34-0-0)
¶ E. Nitrogen nutrient management
- Little difference in yield response between nitrogen sources when properly applied at equivalent rates
- Nitrogen fertilizers require soil incorporation by tillage or precipitation/irrigation amount greater than 0.5 inch
- Ammonia-based fertilizers
- Anhydrous ammonia is 85% liquid and 15% vapor when transported and applied by soil injection
- Converts to 100% vapor after injection
- NH3 vapor dissolves in soil water; converted to NH4+ over time
- Urea-based and ammonium-based fertilizers
- Require soil incorporation soon after application to avoid ammonia volatilization loss
- Nitrate-based fertilizers
- Can be mobilized by precipitation or tillage
- Ammonia-based fertilizers
- Anhydrous ammonia placement
- Ammonia is liquid; must be soil injected; converts to vapor; diffuses outward from injection point immediately after application
- Minimum application depth depends on soil conditions, rate, and application spacing
- Generally applied at depths greater than 4 inches
- Standard depth for row crops about 6 inches in most soils; deeper in coarse-textured soils
- Conditions favoring application losses
- Wet, clayey soils: injection slits don't close or seal
- Coarse texture: greater ammonia diffusion through macropores, especially when dry
- Cloddy or “slabby” soil condition: greater NH3 diffusion through voids formed by tillage
- Dry soil
- Injection slits don't close; voids remain
- Conversion of volatile NH3 to ecchangeable NH4+ requires soil moisture
- Low organic matter: less NH4+ retention
- Wider knife spacing
- Ammonia rate more concentrated; more nitrogen per linear foot of travel
- Diffuses farther from injection point
- Shallow injection places application point too close to soil surface; allows upward diffusion
- Favorable loss conditions are also favorable seedling injury conditions
- Urea/ammonium-based fertilizer placement
- Avoid volatile loss as gaseous ammonia
- Unincorporated, broadcast surface applications expose fertilizers to greater loss potential
- Loss potential ceases with soil incorporation
- Surface banding or streaming can reduce losses
- Avoid placement directly with seed or close to seed
- Conditions favoring application losses
- Higher temperatures (over 60°F)
- “Drying” conditions
- Moist soil surface and rapid evaporation rates
- Snow cover with sunny days above freezing
- Calcareous soils, alkaline pH
- Shifts solution equilibria from NH4+) to NH3
- Sandy, low organic matter soils
- Less retention of NH4+ on CEC
- Less buffering of pH changes
- Crop residues, pastures, sod form “thatch” layer
- Maintains moist soil surface
- Reduces soil/fertilizer contact and movement into soil
- Avoid volatile loss as gaseous ammonia
- Apply fall nitrogen when soil temperatures will stay below 50°F (air temperature below 40°F)
- Nitrification rate very slow below 50°F
- Nitrogen retained in exchangeable ammonium form; avoids leaching losses
- Nitrification inhibitors
- Used to limit potential denitrification or nitrate leaching losses
- Common products: nitrapyrin and DCD (dicyandiamide)
- Inhibits ammonium-to-nitrite step of nitrification
- Toxic to Nitrosomonas
- Restricts nitrification by about one to four weeks
- Effectiveness depends on rate, weather/soil conditions
- Most useful for fall or early spring application, poorly drained soils, or sandy soils
- Not cost effective when leaching or denitrification loss potential is low
- Used to limit potential denitrification or nitrate leaching losses
- Urease inhibitors
- Used to limit ammonia volatilization losses
- Common product: NBPT (N-(n-butyl) thiophosphoric triamide)
- Inhibits activity of urease
- Urease is enzyme responsible for hydrolysis step in conversion of urea to ammonium
- Urease found in soil and on crop residues
- May restrict hydrolysis for about 7 to 14 days
- Widens time frame for effective soil incorporation
- Not effective if urea is soil incorporated or when volatilization loss potential is low
- Used to limit ammonia volatilization losses
- Coated fertilizers
- Useful where there is high potential for leaching and/or volatilization
- Polymer or sulfur/wax coating on fertilizer limits soil/moisture contact; restricts prill from dissolving
- Delays fertilizer release into soil solution
- Thickness and characteristics of coating affect length of delay
- Can replace need for multiple nitrogen applications
- Traditionally used on high value crops, turf, and ornamentals, but more recently on grain crops
- Slow-release, urea-based fertilizers
- Urea-formaldehyde and methylene urea
- Reacting urea and formaldehyde forms polymers (molecular chains) of different lengths
- Nitrogen is released as urea-polymer breaks down
- Polymer chain length determines release rate
- Designed to release nitrogen for 8 to 12 weeks
- Urea-polymer may be mixed with normal urea to provide both fast- and slow-release nitrogen
- Reduced potential for foliar burn
- Urea released slowly as polymer breaks down
- Urea-formaldehyde and methylene urea