⇦ Back to Soil Fertility and Plant Nutrition Home
¶ Introduction
Ion exchange is an important mechanism in soil and water management and in plant nutrition management. This Crop File discusses basic ion exchange concepts.
¶ A. Ions, Solutes, Solutions
- Definitions
- Ion: atom or molecule having a positive or negative charge
- Cation: positively-charged ion
- Anion: negatively-charged ion
- Solute: substance added to solvent to form solution.
- May be solid, liquid, or gas.
- Solvent: substance that dissolves solute during formation of solution.
- Most are liquid, but some might be gas or solid.
- Solution: homogeneous mixture consisting of solute dissolved into solvent.
- Aqueous solution: solution with water as solvent.
- Soil solution: aqueous solution held in soil pores and on soil particle surfaces various solutes including soluble mineral, organic substances, and gases.
- Opposite charges attract; like charges repel.
- Positively charged cations will be attracted to negatively charged anions or to negatively charged surfaces.
- Negatively charged anions will be attracted to positively charged cations or to positively charged surfaces.
- Compounds typically have no charge, but may dissociate in solution as ions.
- Ions are represented by chemical formula and superscript with degree of charge.
- May be monovalent (single charge), divalent (two charges), trivalent (three charges), or more.
- Charge may be either positive or negative.
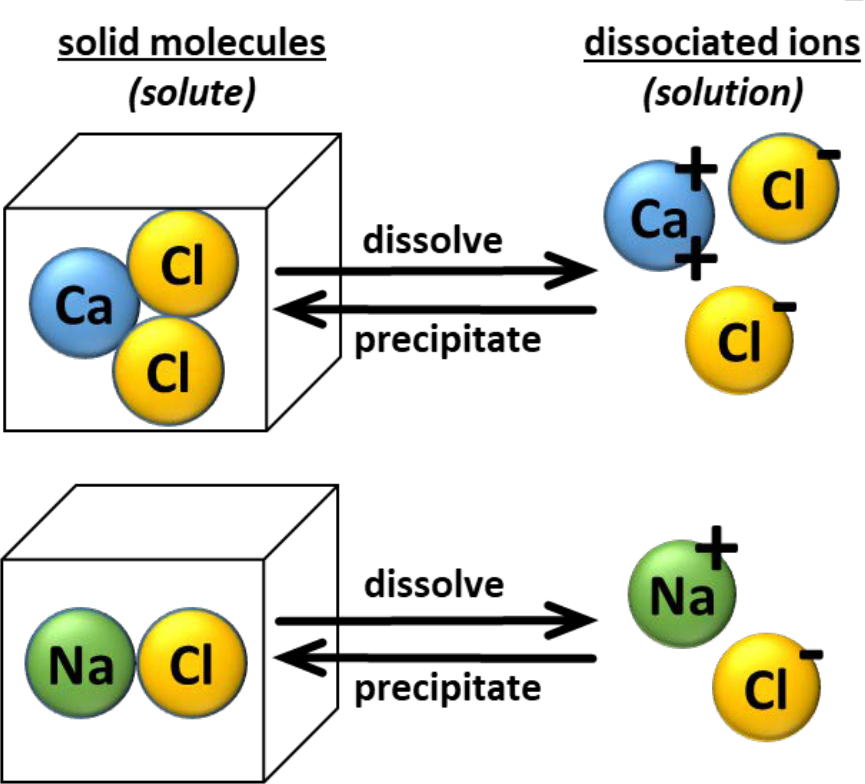
- Example of dissociation (see Figure 1).
- Sodium chloride (NaCl, table salt) and calcium chloride (CaCl2) molecules are present in nature as component of crystalline solid.
- Have no charge.
- When dissolved in water:
- Solid sodium chloride dissociates into monovalent sodium ion with single positive charge (H+) and monovalent chloride ion with negative charge (Cl-)
- Solid calcium chloride dissociates into single divalent calcium ion with two positive charges (Ca++) and two monovalent chloride ions each with one negative charge (two Cl- ions).
- As water evaporates, ions in solution become more concentrated.
- At certain point, charged ions in solution precipitate (crystallize) and associate as solid molecules having no charge.
- Sodium chloride (NaCl, table salt) and calcium chloride (CaCl2) molecules are present in nature as component of crystalline solid.
- Cation(s) will reassociate with anion(s), depending on their charge (valence).
- Number of charges will balance.
- Ions are represented by chemical formula and superscript with degree of charge.
¶ Figure 1. Example: Molecules Dissociating to Ions
¶ B. Ion Exchange
- Definitions
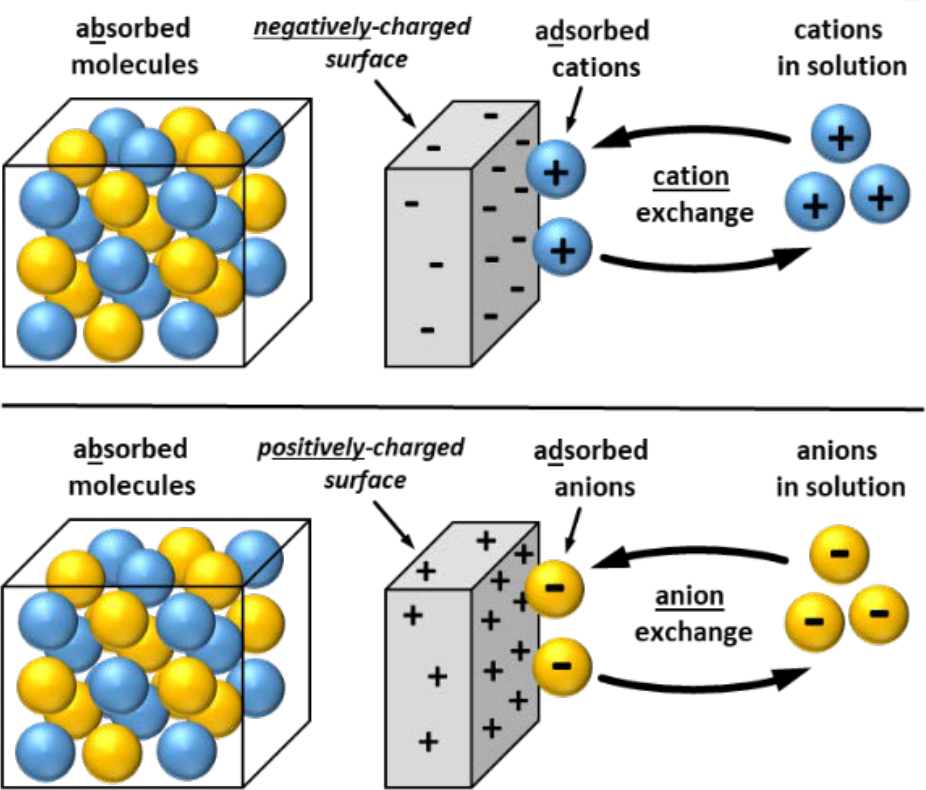
- Ion exchange: exchange of ions of same charge between insoluble solid and the solution in contact with it.
- Adsorb (with “d”): molecules accumulate at solid surface rather than in bulk of solid or liquid material
- Ion concentration at surface is difference than concentration within bulk material.
- Absorb (with “b”): molecules are assimilated throughout bulk of solid or liquid material
- Ion concentration is same throughout bulk material
- Colloid: substance consisting of particles substantially larger than atoms or ordinary molecules but too small to be visible to unaided eye.
- Soil colloids are particles of 2 micrometers or smaller.
- Soil colloids are primarily clay and humus particles.
- Have small size, but large specific surface.
- “Plants don’t eat their food, they drink it.”
- Plants absorb nutrients as ions, not as solids.
- Solid plant nutrient compounds must be converted to ionic forms to be taken up by plant root systems (see Table 1).
- Agronomically, ion exchange refers to movement of cations and anions within soils.
- Ion exchange occurs primarily between soil colloid surfaces and soil solution.
- Ion exchange occurs between soil solution and surface of root hairs.
- Solid surfaces must have net negative charge to adsorb cations or net positive charge to adsorb anions (see Fig. 2).
- Relative ability to adsorb cations or anions referred to as “exchange capacity”.
- Ions in solution can exchange with ion or ions of equivalent charge that are adsorbed on exchange surface.
- Exchange capacity affected by chemistry and physical factors.
- Relative ability to adsorb cations or anions referred to as “exchange capacity”.
- Cation exchange capacity is important soil property (see Crop File 4.01.010, Cation Exchange Capacity basics).
¶ Figure 2. Ion Exchange; Absorb vs Adsorb
¶ Table 1. Common Ions Found in Soil Solution |
||
|---|---|---|
| Element | Cations ion name, formula/charge |
Anions ion name, formula/charge |
| Aluminum | aluminum, Al+++ | |
| Boron | borate, H2BO3 | |
| Calcium | calcium, Ca++ | |
| Carbon/Oxygen | carbonate, CO3- bicarbonate, HCO3- |
|
| Chlorine | chloride, Cl- | |
| Copper | copper, Cu++ | |
| Hydrogen | hydrogen, H+ | |
| Iron | ferrous ion, Fe++ reduced iron or "iron(II)" ferric ion, Fe+++ oxidized iron or “iron(III)” |
|
| Magnesium | magnesium, Mg++ | |
| Manganese | manganic ion, Mn++ manganous ion, Mn+++ |
|
| Nitrogen | ammonium, NH4+ | nitrate, NO3- nitrite, NO2- |
| Phosphorus | phosphate, PO4- monohydrogen phosphate, HPO4- dihydrogen phosphate, H2PO4- |
|
| Potassium | potassium, K+ | |
| Sodium | sodium, Na+ | |
| Sulfur | sulfate, SO4- | |
| Zinc | zinc, Zn++ | |
¶ References:
Tisdale, S.L., et. al. 1993. "Basic Soil-Plant Relationships" in Soil Fertility and Fertilizers. MacMillan Pub. Co., New York. pp. 80-94.