⇦ Back to Soil Fertility and Plant Nutrition Home
¶ Introduction
This Crop File discusses the concept of “base saturation” and its relationship with cation exchange capacity (CEC) and soil pH. Other Crop Files have background information on this topic and use of base saturation in nutrient management,
¶ A. Soil Cations
- Cations are found on soil colloid exchange sites and in soil solution
- Cation composition on exchange complex reflects soil solution composition
- Ions (cations and anions) develop equilibrium between soil solution and exchange complex
- When either solution or exchange complex are changed, other will eventually come into equilibrium
- Primary soil cations essential to plant growth:
- Ammonium, NH4+
- Typically converted to nitrate (NO3-) for plant uptake.
- Potassium, K+
- Calcium, Ca2+
- Magnesium, Mg2+
- Ammonium, NH4+
- Soil cations that affect soil pH:
- Hydrogen, H+
- Aluminum, Al3+
- Sodium, Na+
- Has indirect effect on increasing soil pH
- Soils are typically above pH 8.0 when sodium is in excess (i.e., “sodic” soil condition)
- Are occasional situations where sodic soils are also acidic
- Other micronutrient soil cations are also found on cation exchange sites.
- Includes Zn2+, Fe2+, Fe3+, Mn2+, Mn3+, Cu2+, Ni2+, Co2+
- Are considered essential for plant growth
- Micronutrient metals only account for very small fraction of total exchangeable cations
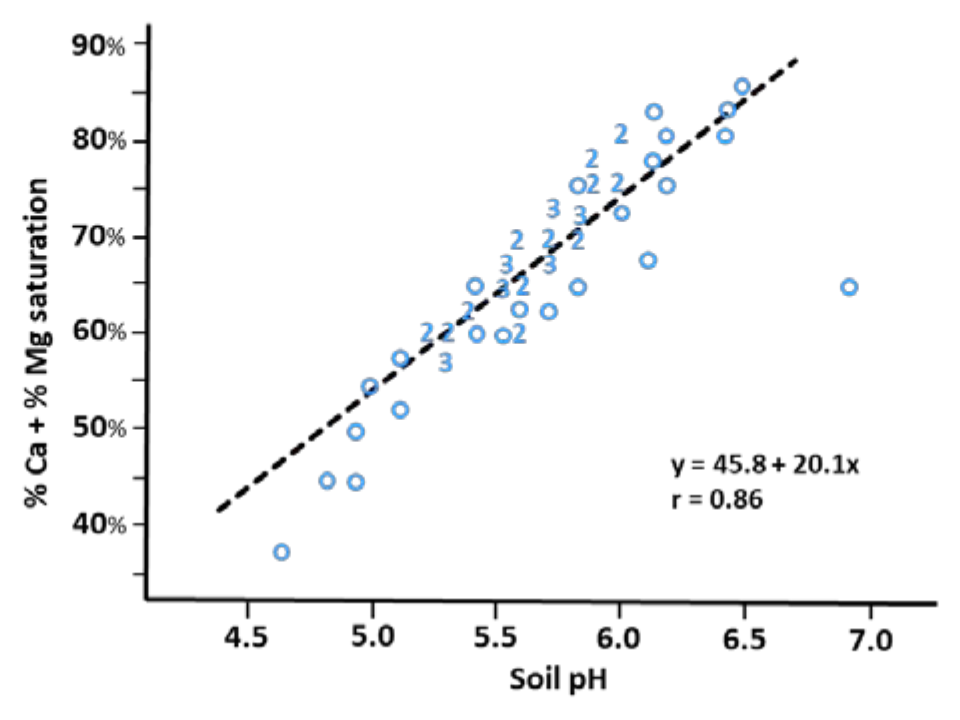
¶ Figure 1. Relationship Between Soil pH and Exchangeable Ca + Mg
¶ B. Base Saturation
- Term to describe proportion of “basic cations” held on soil exchange sites.
- Base Saturation (%) = (Base cations/CEC) x 100
- Base saturation of “60%” means that 60% of soil exchange sites are occupied by base cations, regardless of CEC
- 60% base saturation = 3.0 mEq/100g of base cations if CEC is 5 mEq/100g
- 60% base saturation = 30.0 mEq/100g of base cations if CEC is 50 mEq/100g
- CEC = Base cations + Acid cations
- Base Cations
- Ammonium, NH4+
- Potassium, K+
- Calcium, Ca2+
- Magnesium, Mg2+
- Sodium, Na+
- Acid Cations
- Hydrogen, H+
- Aluminum, Al3+
- CEC = (Ca2+ + Mg2+ + K+ + Na+) + (H+ + Al3+ + NH4+)
- Base saturation increases as soil pH increases
- Base saturation is essentially 100% if base cations are held on all cation exchange sites
- Neutral to alkaline soils (pH > 6.5)
- Low Al3+ and H+
- High Ca2+, Mg2+, K+, & Na+
- Soils above pH 7 or above often considered 100% base saturated because there are few or no exchangeable acid cations
- Acid cations H+ and Al3+ become more prevalent as pH decreases and soil becomes acidic
- Base cations replaced by acid cations as pH decreases, so base saturation decreases (see Figure 1)
- Al is actually present as Al(OH)2+
- Base Cations
¶ C. Calculating Base Saturation
- CEC by summation is calculated estimate based on routine soil test levels.
- Exchangeable K, Ca, Mg, and Na typically determined from ammonium acetate or Mechlich-3 extraction.H+ concentration is estimated by buffer pH value to estimate concentration of acidic cations
- Exchangeable ammonium (NH4+) and aluminum (Al3+) not often analyzed. ii. NH4+ concentrations usually quite low, so not routinely included in summation CEC
- Exchangeable K, Ca, Mg, and Na typically determined from ammonium acetate or Mechlich-3 extraction.H+ concentration is estimated by buffer pH value to estimate concentration of acidic cations
- Base Saturation (%) = (base cations ÷ CEC) x 100
- Convert base cation concentrations from “ppm” to “mEq/100g”.
- Divide each cation by appropriate equivalent weights.
- K = 390
- Ca = 200
- Mg = 120
- Na = 230
- Divide each cation by appropriate equivalent weights.
- Calculate acid cation concentration as “mEq/100g” using buffer pH value
- H mEq/100g = (7 – buffer_pH) x 12
- Add base cation and acid cation concentrations as mEq/100g to calculate “CEC by summation”
- Add base cation concentrations as mEq/100g, divide sum by CEC value, and multiply by 100 to calculate base saturation percentage (see Table 1). i. Soil #1: pH = 5.0, 53% base saturation ii. Soil #2, pH = 7.7, 100% base saturation (no acidity)
- Convert base cation concentrations from “ppm” to “mEq/100g”.
- Cation saturation (%) = (individual cation ÷ CEC) x 100
- Divide individual cation concentrations (as mEq/100g) by CEC value, then multiply by 100 to calculate individual cation saturation percentages (see Table 1).
- Soil #1: Ca = 1471 ppm ÷ 200 = 7.4 mEq/100g
- (7.4 ÷ 20.4) x 100 = 36% Ca saturation
- Soil #2: Ca = 4639 ppm ÷ 200 = 23.2 mEq/100g
- (23.2 ÷ 29.0) x 100 = 80% Ca saturation
- Soil #1: Ca = 1471 ppm ÷ 200 = 7.4 mEq/100g
- Divide individual cation concentrations (as mEq/100g) by CEC value, then multiply by 100 to calculate individual cation saturation percentages (see Table 1).
- Calculating cation ratios
- Ratios are calculated on charge equivalent basis.
- Convert base cation concentrations from “ppm” to “mEq/100g”.
- Divide each cation by appropriate equivalent weight
- Divide first cation concentration (as mEq/100g) by second cation concentration (as mEq/100g), then add “:1: to result (see Table 2)
- Soil #1: Ca ÷ Mg = 7.4 ÷ 2.3 = Ca/Mg 5.2:1
- Soil #2: Ca ÷ Mg = 23.2 ÷ 1.0 = Ca/Mg 23.1
¶ Table 1. Example Calculations, Base Saturation Percentage and Individual Cation Saturation Percentages |
||||
|---|---|---|---|---|
| Soil #1 Test Result | Equivalent Weights |
mEq/100g | Saturation % | |
| pH | 5.0 | --- | --- | --- |
| K ppm | 417 | ÷ 390 = | 1.1 | 5 |
| Ca ppm | 1471 | ÷ 200 = | 7.4 | 36 |
| Mg ppm | 274 | ÷ 120 = | 2.3 | 11 |
| Na ppm | 13 | ÷ 230 = | 0.0 | 0 |
| BpH | 6.2 | (7 – BpH) x 12 = | 9.6 | 47 |
| CEC by Summation = | 20.4 | |||
| Sum of Base Cations = | 10.8 | |||
| Base saturation - (base cations / CEC) x 100 = | 53% | |||
| Soil #2 Test Result | Equivalent Weights |
mEq/100g | Saturation % | |
| pH | 7.7 | --- | --- | --- |
| K ppm | 397 | ÷ 390 = | 1.0 | 4 |
| Ca ppm | 4639 | ÷ 200 = | 23.2 | 80 |
| Mg ppm | 536 | ÷ 120 = | 4.5 | 15 |
| Na ppm | 67 | ÷ 230 = | 0.3 | 1 |
| BpH | 0 | (7 – BpH) x 12 = | 0.0 | 0 |
| CEC by Summation = | 29.0 | |||
| Sum of Base Cations = | 29.0 | |||
| Base saturation - (base cations / CEC) x 100 = | 100% | |||
¶ Table 2. Example, Calculating Cation Ratios |
||||
|---|---|---|---|---|
| Soil #1 | Ca | Mg | K | |
| Ratio | ----- mEq/100g ----- | |||
| Ca:Mg | 3.2 : 1 | 7.4 | 2.3 | --- |
| Ca:K | 6.7 : 1 | 7.4 | --- | 1.1 |
| Mg:K | 2.1 : 1 | --- | 2.3 | 1.1 |
| Soil #2 | Ca | Mg | K | |
| Ratio | ----- mEq/100g ----- | |||
| Ca:Mg | 5.2 : 1 | 23.2 | 4.5 | --- |
| Ca:K | 23.2 : 1 | 23.2 | --- | 1.0 |
| Mg:K | 4.5 : 1 | --- | 4.5 | 1.0 |
¶ D. Soil Types and Base Saturation
- Many soils in Great Plains and western area have high base saturations.
- Ca2+ typically dominates in soil solution, also dominates exchange complex
- Soils in region formed from materials with relatively high base cation concentrations; e.g., feldspars, carbonates, etc
- Low rainfall minimized amount of mineral weathering and cation leaching over time, so cations remained in upper soil profile
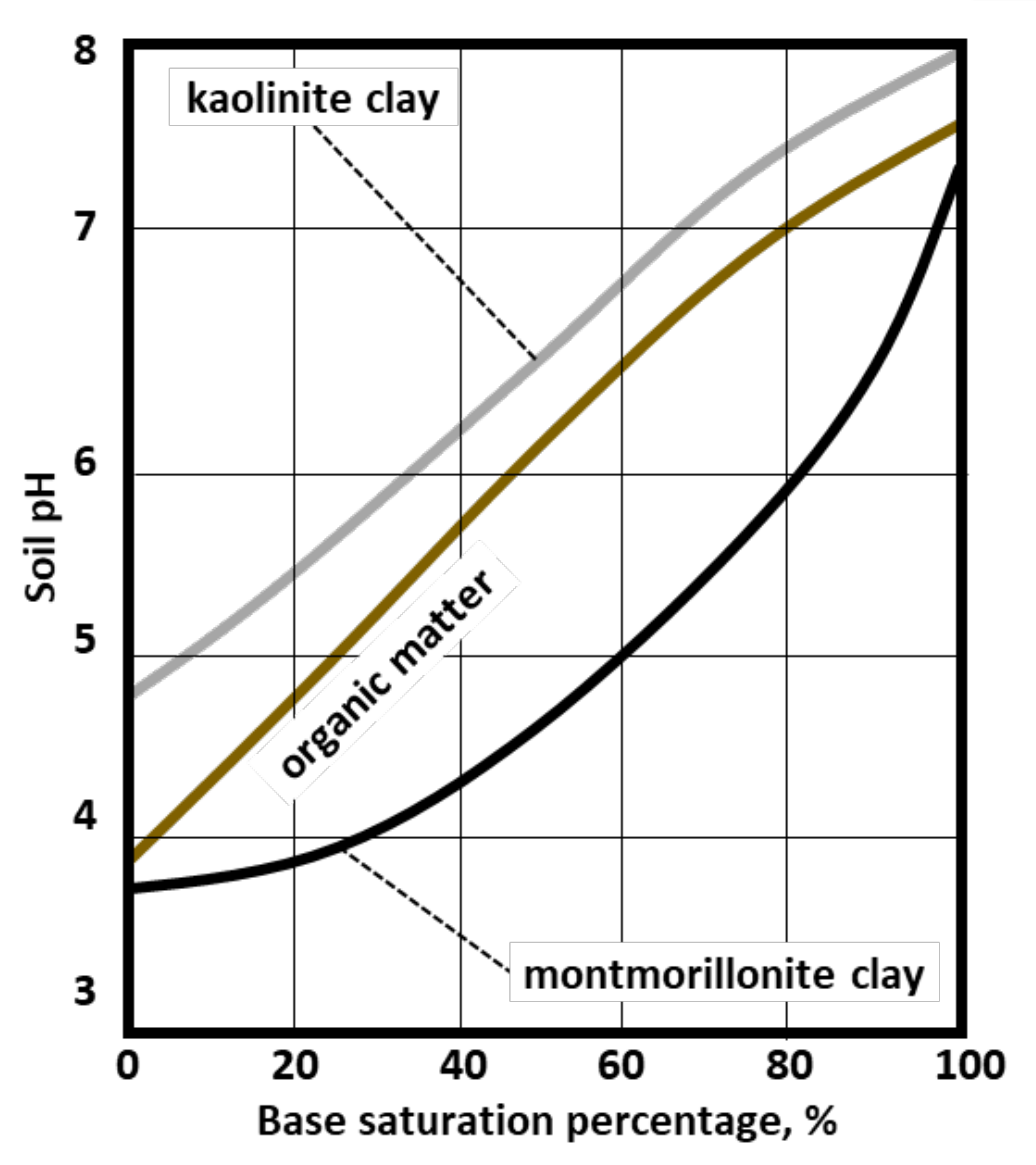
- Soils are dominated by less weathered clay minerals (e.g., montmorillonite) with high CEC
- Soils have been farmed for comparatively short time, so fewer base cations removed by crop harvest
- Highly weathered and/or acidic soils tend to have low base saturation
- Soils have highly weathered clays with low CEC (e.g., kaolinite)
- Cations are not bound as tightly and more easily removed from exchange surfaces
- High rainfall responsible for increased weathering which releases cations
- Released cations are leached from upper soil profile by heavy annual rainfall (e.g., humid southeast U.S., tropical areas
- Cations are replaced by acidic cations, especially H+ and Al3+
- CEC becomes more pH dependent as soils become more acidic
- Ratio for humid temperate region soil (within pH range of 5 to 6) is change of roughly 5% base saturation for each 0.1 unit change of soil pH
- Soils have highly weathered clays with low CEC (e.g., kaolinite)
¶ Figure 2. Base Saturation Relationships for Soils with Different Sources of CEC
¶ References
Foth, H.D. 1984. “Soil Chemistry” in Fundamentals of Soil Science, 7th ed.
John Wiley & Sons, New York. pp. 198-201.
Tisdale, S.L., et. al. 1993. "Basic Soil-Plant Relationships" in Soil Fertility and Fertilizers. MacMillan Pub. Co., New York. pp. 91-93.