⇦ Back to Soil Fertility and Plant Nutrition Home
¶ Introduction
Calcareous soils are most commonly found in arid and semi-arid regions of the western U.S., but also occur intermittently in other areas. The terms “calcareous”, “alkaline”, and “alkali” are sometimes used interchangeably, but are different and have specific meanings.
¶ Definitions
The Soil Science Society of America defines a calcareous soil as a “soil containing sufficient free calcium carbonate (CaCO3) and other carbonates to effervesce visibly or audibly when treated with cold 0.1M HCl”. Calcareous soils have usually contain 1% or greater CaCO3 equivalent.
An alkaline soil is one with a soil pH above 7.0. Calcareous soils are usually alkaline, but not all alkaline soils are calcareous.
“Alkali” is an older term used to describe a high pH soil with excess sodium. The modern term is “sodic” which the SSSA defines as a “soil containing sufficient exchangeable sodium to adversely affect crop production and soil structure under most conditions of soil and plant type”.
¶ “Excess Lime” Test
The Servi-Tech Laboratory routinely includes “excess lime” as a determination included with the soil pH analysis. “Free lime” or “reactive carbonates” are terms that might substitute for the term excess lime.
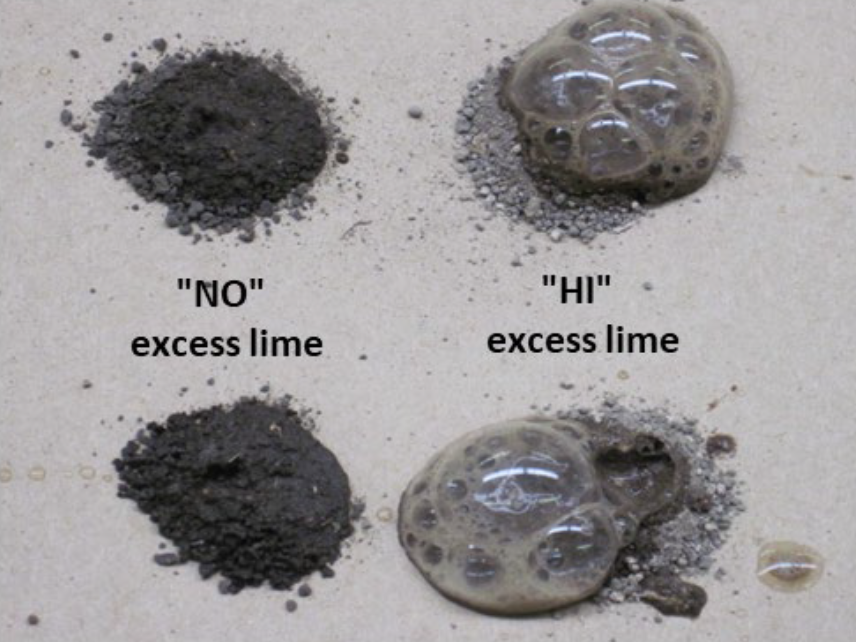
Excess lime is a qualitative test measured by adding a drop or two of 1 N hydrochloric acid to a small amount (about one-half teaspoon) of soil, then observing the relative amount of effervescence or fizzing (see Fig. 1). The effervescence results from the reaction of the acid with the soil carbonates, resulting in formation of carbon dioxide bubbles.
The analyst determines a subjective, visual rating from the relative intensity of the reaction (see Fig. 2). A “NO” rating means no effervescence was noted. A “LO” rating means that effervescence was observed, but limited (i.e., a few bubbles, little fizzing). A “HI” rating means the effervescence was vigorous with a quick reaction and lots of fizzing.
¶ Figure 1, Determining Effervescence Using HCl

¶ Figure 2. High pH, Alkaline Soils. Non-Calcareous Alkaline Soil to Left. Calcareous, Alkaline Soil to Right
¶ Total Carbonates
The Soil Science Society further defines a calcareous soil as containing from 1% to almost 100% carbonates. Calcium carbonate (CaCO3) is typically the dominant form of carbonate, but soils may contain magnesium carbonate (MgCO3) and other carbonates, depending on the original material from which the soil was formed.
“Total carbonates” can be determined by treating a weighed soil sample with a concentrated acid that reacts with the soil carbonates. After a certain time, the sample is weighed to measure how much of the original sample was lost as carbon dioxide during the reaction. The difference in weight is expressed as the percent total carbonate.
¶ Reactive carbonates
A soil carbonate or lime content of roughly 2% is the point at which a trained analyst will start to see effervescence or fizzing. The respective amount of effervescence or excess lime rating depends on the size of the carbonate particles rather than the total carbonate content.
Two different soils with the same total carbonate content may have “NO” and “HI” excess lime ratings. Very fine lime particles react more rapidly than do coarse lime particles because of the greater surface area that is available to react with the acid solution.
Soils with a “HI” excess lime rating have a large proportion of very fine lime or carbonate particles which react quickly with the applied acid. Soils with a “NO” excess lime rating have either a very low total carbonate content or the carbonate particles are large with a much lower reactive surface area.
Finer particles have more surface area per unit of weight than coarser particles. A one-inch cube of limestone - measuring one inch along each side) - weighs 1.57 ounces. The cube has six surfaces: top, bottom, and four sides. Each surface is one square inch, so it has a total surface area of 6 square inches.
Assume the same one-inch cube were sliced into smaller cubes of 1/100th inch (0.01 in.) along each side. We would now have one million very small cubes (100 * 100 * 100 = 1,000,000).
Each of the smaller cubes would have a total of 6/10,000th square inch of surface area (0.01 sq. in. * 0.01 sq. in. * 6 sides = 0.0006 sq. in.). The original one-inch cube would have yielded 600 sq. in of surface area (0.0006 * 1,000,000 = 600 sq. in.), but would still weigh 1.57 oz.
¶ Figure 3: Sorghum x Sudan Growing in a Calcareous Soil Showing Symptoms of Iron Deficiency Chlorosis

https://pubs.nmsu.edu/_a/A151/l
¶ Agronomic Concerns: Iron Deficiency Chlorosis (IDC)
IDC is a common problem that often occurs when susceptible plant varieties are grown on calcareous soils (see Fig. 3). The symptoms of iron deficiency chlorosis are prominent green veins with mild to severe yellowing (“chlorotic”) leaf tissue between the veins. This can lead to leaf death, reduced plant vigor, and possibly plant death.
We do not fully understand the exact causes and mechanisms involved in iron deficiency chlorosis. Weather conditions and other soil factors contribute to the onset of iron chlorosis. Soybeans, sorghums, and sugarbeets are susceptible to iron chlorosis. Many ornamentals, shrubs, and trees (e.g., pin oak, redbud) are also susceptible. Bluegrass and certain turf varieties are susceptible to chlorosis.
The excess lime test is a simple, quick way to screen soils for their iron chlorosis potential. Iron chlorosis symptoms may occur in plants growing on soils with a “HI” and possibly a “LO” excess lime rating. Chlorosis symptoms would not be expected to develop in soils with “NO” excess lime in the surface. Chlorosis could occur in these soils if excess lime is present deeper in the crop root zone.
If feasible, soils can be treated with proper rates of acid-forming amendments like elemental sulfur or aluminum sulfate. Switching to more chlorosis tolerant varieties or plant species may be a more economical method of dealing with chlorosis. Avoid using chlorosis sensitive crops or varieties in areas with HI excess lime.
¶ Agronomic Concerns: Nutrient Management
The solubility and the subsequent plant availability of some nutrients, like phosphorus and zinc, are strongly affected by soil pH. In calcareous soils, phosphorus may form less soluble calcium and magnesium phosphates in calcareous soils. Zinc may form less soluble zinc oxides and carbonates. Placing fertilizers in concentrated bands or zones when applying phosphate or zinc materials to calcareous soils may be beneficial. This method helps minimize the soil contact with the fertilizer and delays the formation of the less soluble nutrient compounds.
¶ Agronomic Concerns: Herbicide Performance
Calcareous soils may affect the performance and persistence of soil-applied herbicides, insecticides, or fungicide, depending on their chemistry. Consult the label for any rate adjustments or replanting restrictions.
¶ References
Soil Science Society of America. accessed 02June2022. Glossary of Soil Science Terms. https://www.soils.org/publications/soils-glossary/
Spiva, C. 1990. Understanding Calcareous Soils.Solution Sheet newsletter. UnoCal Chemicals Division, El Seundo, CA. 4 pg..
USDA-NRCS. January 1998. Soil Quality Indicators: pH. Soil Quality Information Sheet. 2 pg.
USDA-NRCS. Soil Survey Technical Note 5: Assessing Carbonates in the Field with a Dilute Hydrochloric Acid (HCl) Solution accessed 02June2022. https://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/ref/?cid=nrcs142p2_053572