⇦ Back to Soil Fertility and Plant Nutrition Home
¶ Key Questions
- Question: “Do I need lime?”
- Answer: “Yes” (if soil pH result less than 6.2)
- Question: “How much lime do I need?”
- Answer: Calculate from buffer pH result
¶ A. Measuring pH
- pH measured in liquid solution
- Solids and gases do not have pH, unless capable of dissolving in water
- If dissolved in water solution, substance may cause dissociation of water molecules
- May contribute either hydrogen ions (H+) or a hydroxyl ions (OH-) to that solution.
- If more water molecules dissociated, more hydrogen or hydroxyl ions are present in solution
- pH will be lower or higher depending on number of ions
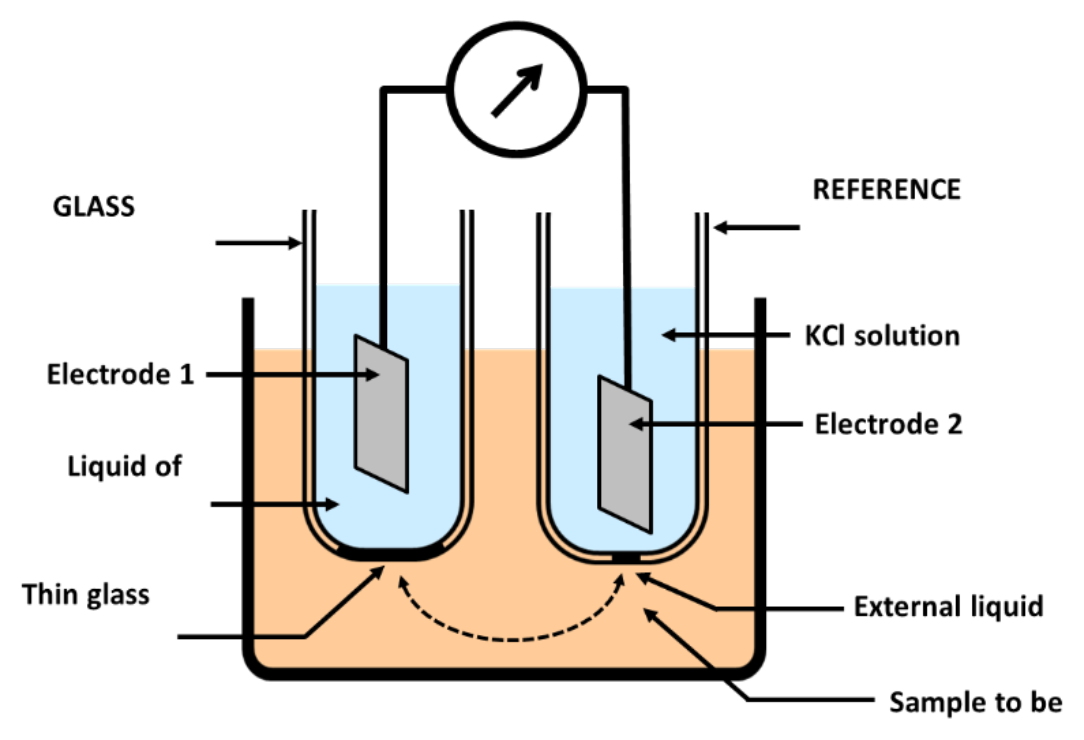
- pH measured with glass electrode
- Electrode is glass shaft with a bulb of special glass membrane
- Membrane is sensitive to variations in pH
- Liquid solution pH changes electrical potential across glass membrane
- Change in electrical potential compared to second “reference” electrode
- Comparative change in voltage between the two electrodes expressed as “solution pH” (see Figure 1)
- Laboratory-grade electrodes can compensate for sensitivity effects
- Can be affected by the solution temperature
- Affected by both type and concentration of ions in solution being measured
¶ Figure 1. Schematic of pH Electrode Working Principle.
¶ B. Determining soil pH
- Soil pH also called “soil reaction”
- Solution chemistry is soil chemistry
- Is not pH of soil solids (sand, silt, clay particles, or organic matter)
- Is pH of soil “solution”
- Soil solution: water held in voids between soil particles with dissolved constitutents
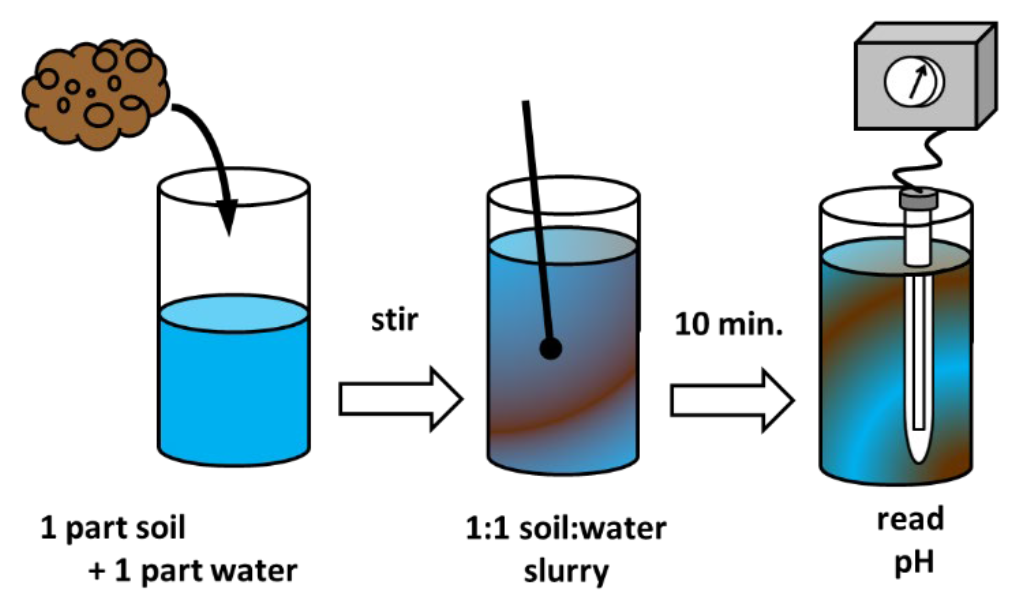
- Soil pH commonly determined in soil/water slurry (see Figure 2)
- Most common ratio is 1 part soil to 1 part water; “1:1 soil:water”
- 5 or 10 gram scoop of soil mixed with 5 or 10 milliliters of deionized water
- Ratio of 1:2, 1:5, or 1:10 soil:water may be used
- High organic matter soils
- Soils with clay types that absorb large amounts of water
- Typically not much difference between ratios
- Important to maintain consistency
- Important to list soil:water ratio when reporting results
- Slurry is stirred allowed to stand for 10 minutes
- pH determined by inserting electrode while slurry is being stirred
- Approximates pH of field soil when saturated with water
- After electrode readings stabilize, units can be reported as “soil pH”, “water pH” or “pHw”
- Most common ratio is 1 part soil to 1 part water; “1:1 soil:water”
- Soluble salts can affect soil/water pH
- Electrode sensitivity affected by both type and concentration of ions in solution being measured
- Can be naturally occurring salts or salts from fertilizer application
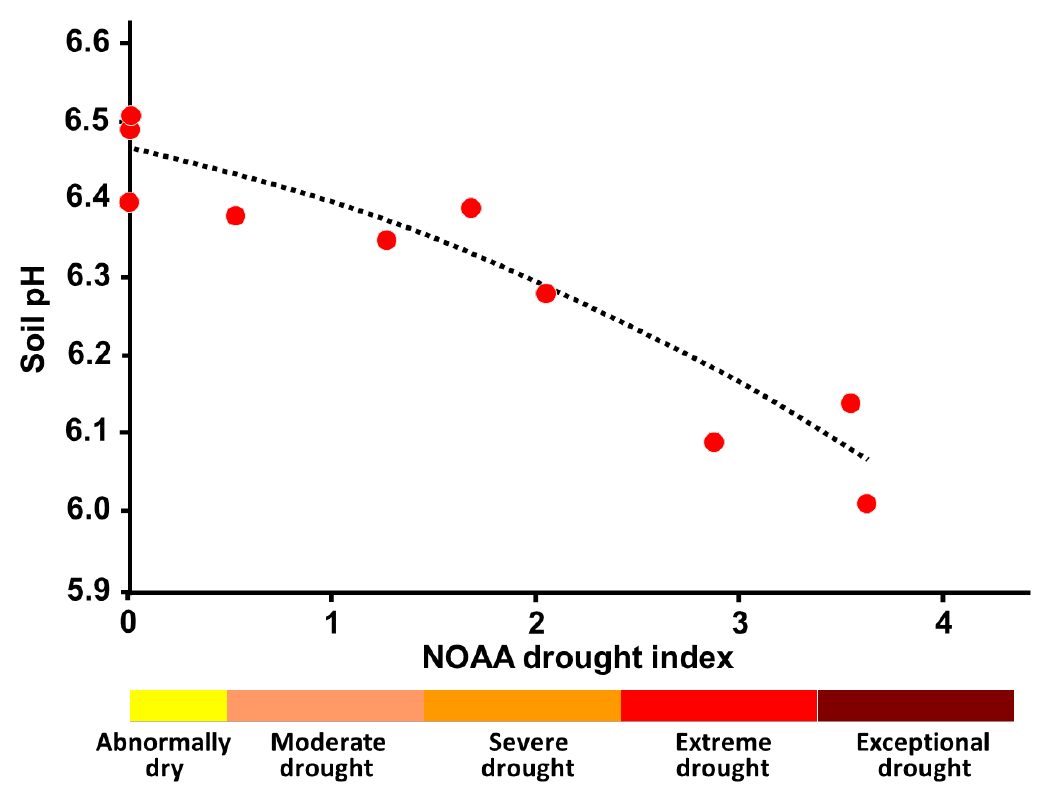
- Result of soil:water pH determination may decrease following extended dry period
- Salts accumulate in soil when weather permits high evaporation rates
- More salts in pH solution may help depress pH
- Air contains carbon dioxide (CO2) which dissolves in soil water to form weak carbonic acid solution
- Less soil water under drought conditions
- More soil pore spaces filled with air rather than water due to dry conditions
- Increased amount of carbonic acid helps depress soil pH result
- Soil pH may be lower when sampled in fall than when sampled in spring
- Soil:water pH may be depressed by 0.3 to 0.5 pH units, or more, following extended drought (see Figure 3)
- pH result typically rebounds when more normal precipitation returns
- Salts accumulate in soil when weather permits high evaporation rates
- pH determined with salt solution can offset dry weather depression effect
- Procedure is same as using deionized water
- Commonly used salts dissolved in deionized water
- Calcium chloride (0.01M CaCl2)
- Potassium chloride (1N KCl)
- Masks differences due to soil salt content
- Helps displace larger percentage of exchangeable H+ and Al3+ than using water
- Reading typically about 0.5 unit lower than deionized water alone
- Helps stabilize electrode more rapidly
- After electrode readings stabilize, units can be reported as “soil pH”, “salt pH” or “pHs”
- May be back-calculated and reported as “water pH equivalent”
¶ Figure 2. Process of Determining Soil pH
¶ Figure 3. Average pH vs Drought Index for 11 Southeastern Kentucky Counties (2007–2009)
¶ C. Active and reserve acidity
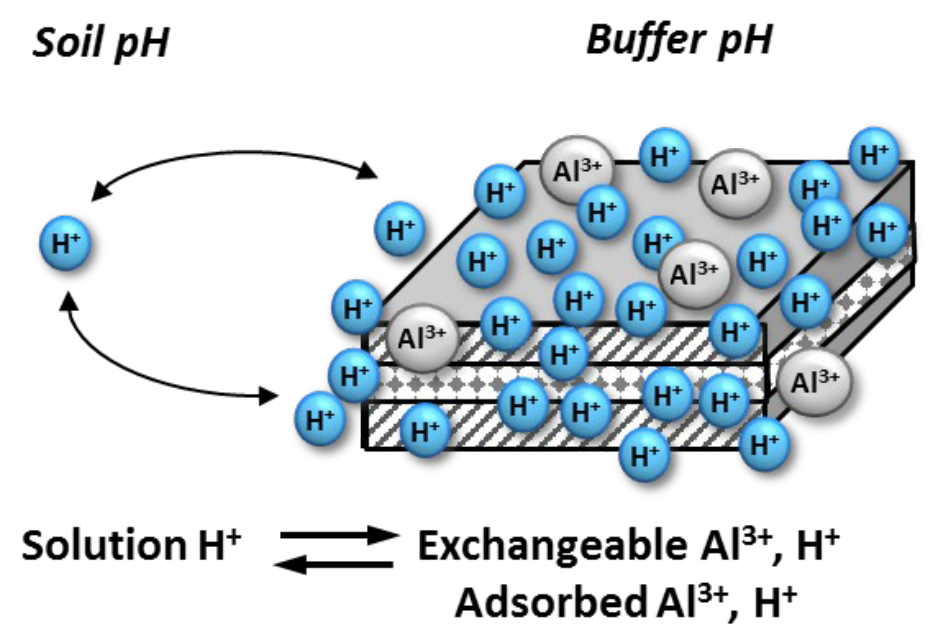
- Three “pools” or “reservoirs” of soil acidity (see Figure 4)
- Active acidity
- Hydrogen ion (H+) concentration of soil solution
- Very small fraction of total soil acidity
- Reserve acidity
- Quantity of acidic cations (H+, Al3+) that occupy sites on exchange complex
- Residual acidity
- Quantity of all bound aluminum and bound hydrogen ions contained in soil minerals or organic matter
- Active acidity
- Soil solution pH (active acidity) is controlling variable
- Affects mineral solubility and dissolution
- Affects nutrient availability
- Governs roots and microorganism environment
- Reserve acidity is able replenish H+ ions removed from soil solution
- For each H+ ion in soil solution are hundreds, thousands of H+ ions on exchange surfaces
- Exchangeable H+ ions are “held in reserve” as reservoir of potential acidity
- Will maintain some level of active acidity
- Buffer capacity is ability to maintain given level of active acidity
- Buffer pH measurement estimates size or capacity of reserve acidity pool
- Size of reserve acidity pool affects buffering capacity
- Cation exchange capacity (CEC) affects buffering capacity
- High clay and/or high organic matter soils have higher potential buffering capacity
- Coarse-textured soils with little to no clay or organic matter have low buffering capacity
¶ Figure 4. Functions of pH Measurements
¶ D. Determining buffer pH and lime requirement
- If initial soil pH result is less than pH 6.2, determine buffer pH (see Figure 5)
- Buffer pH solution at pH 7.5 added to soil:water slurry
- Soil:water:buffer slurry is stirred
- Slurry stands for 30 minutes so buffer solution can react with reserve acidity and reach equilibration point
- pH of soil:water:buffer slurry determined by inserting electrode
- After electrode readings stabilize, units are reported as “buffer pH”
- Result includes notation of buffer method used
- Lime requirement calculated from buffer pH result
- Buffer solution formulated at pH 7.5
- Equilibrates with reserve acidity while allowed to stand (i.e., 30 minutes)
- Difference between original soil pH and buffer pH is amount of soil acidity that must be neutralized
- Lime rate calculation based on unit change from initial buffer solution pH (7.5) and final pH of soil:water:buffer slurry after equilibration period
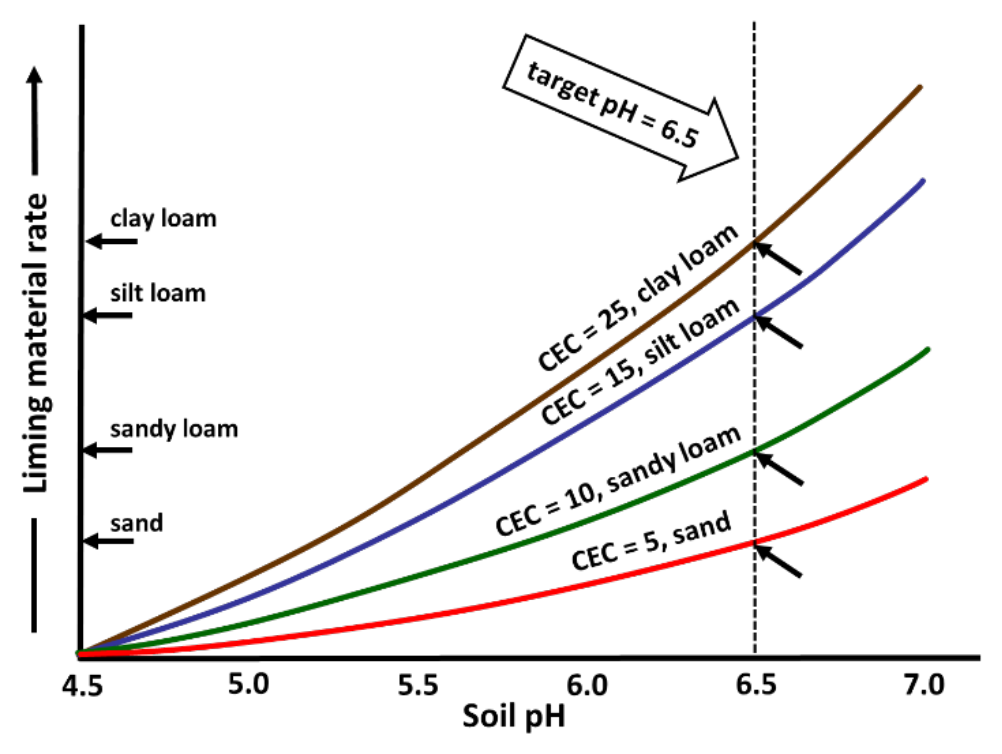
- Buffering capacity affects lime rate required to reach target rate (see Figure 6)
- Higher rates needed for high clay and/or high organic matter soils
- Lower rates needed for coarse-textured soils with little to no clay or organic matter
- Liming material requirement typically suggested as units of pure calcium carbonate (CaCO3)
- Most common liming materials are calcium and/or magnesium oxides, hydroxides, carbonates, and silicates
- “Ag lime” is common source of calcium carbonate
- Calcium or magnesium cations do not neutralize acidity
- Associated anion(s) react with H+ ions and neutralize acidity
- Must convert neutralizing value of recommended rate of pure CaCO3 to equivalent weight of other material
- Most common liming materials are calcium and/or magnesium oxides, hydroxides, carbonates, and silicates
- Lime rate is amount to be applied in one application to reach desired soil pH target
- Target pH is typically at midpoint of pH range for optimum crop growth
- 6.5 or 6.8 is common target pH
- Is within range of optimal availability for many nutrients and many crops
- Target pH of 5.5, 6.0, or 7.0 might be required for specific situations
- Lime rate adjustments
- Liming material type and quality
- Includes chemical and physical qualities
- Typically must be registered with state agency
- Includes chemical quality: pure material (carbonate) vs. foreign material (dirt sand, etc.)
- Includes physical quality (fineness, particle size)
- Lime quality terminology may differ between states, but conveys similar meaning
- ECC = effective calcium carbonate
- ECCE = effective calcium carbonate equivalent
- RNV = relative neutralizing value
- ENM = effective neutralizing material
- ENP = effective neutralizing power
- ELM = effective liming material
- NI = neutralizing index
- Sampling depth
- May differ from standard laboratory depth
- e.g., 6-inch sample with 8-inch standard
- [6 in. / 8 in.] = 0.75 of recommended rate
- Rate may already be adjusted in laboratory recommendations
- Mineral vs. organic soils
- Organic soil: 10% organic matter or greater
- Preparation for analysis may be different
- Interpretations may be different
- Liming material type and quality
¶ Figure 6. Typical Relationship Between Soil Texture and Cation Exchange Capacity (mEq/100g) for Adjusting Lime Rate to Reach Target Soil pH
¶ E. Buffer pH methods
- Method requirements
- Buffer solutions have large capacity to resist pH change
- Typically include weak acid and salt of that same weak acid
- Solution neutralizes both acids and bases
- Short equilibration time (15 to 30 minutes)
- Are calibrated against titration with standard solution and laboratory incubation studies
- Buffer solutions have large capacity to resist pH change
- Woodruff buffer
- First buffer pH method; developed 1948
- Adapted for low CEC soils having low lime requirement
- Contains hazardous material
- Adams-Evans buffer
- Originally developed for low CEC, low organic matter soils of Southeast and Mid-Atlantic regions
- Adapted for soils with lime requirement less than 2 ton/ac
- May overestimate lime requirement
- Contains hazardous material
- Mehlich buffer
- Calibrated to crop yield, not to target pH
- Will generally recommend lime to achieve soil pH slightly above 5.5
- Best suited for lower CEC soils and Histosols
- May overestimate lime requirement for coarse textured soils
- Contains hazardous material
- SMP buffer
- SMP = Shoemaker-McLean-Pratt
- Best adapted for fine to medium textured soils
- Originally used in North Central and Northeast US
- Underestimates lime requirements in soils with CEC less than 7 mEq/100g
- Most accurate with lime rates over 2 tons/ac
- Contains hazardous material
- Sikora-1 buffer
- Mimics performances of SMP buffer
- Uses same lime rate calculations as SMP buffer
- Does not contain hazardous material
- Sikora-2 buffer
- Incorporates salt pH reading with KCl solution
- Not affected by free salts in soil
- Electrode stabilizes rapidly; better performance in sandy soils
- Does not contain hazardous material
¶ References:
Tisdale, et. al. 1993. Soil Fertility and Fertilizers (5th ed.). MacMillan Publishing, New York. pg. 189-204, 364-378.
Sikora & Howe. 2010 presentation on 1 M KCl soil pH method – Soils. soils.rs.uky.edu/technical_Info/NewPHmethod.ppt accessed 12Sept2016