⇦ Back to Soil Fertility and Plant Nutrition Home
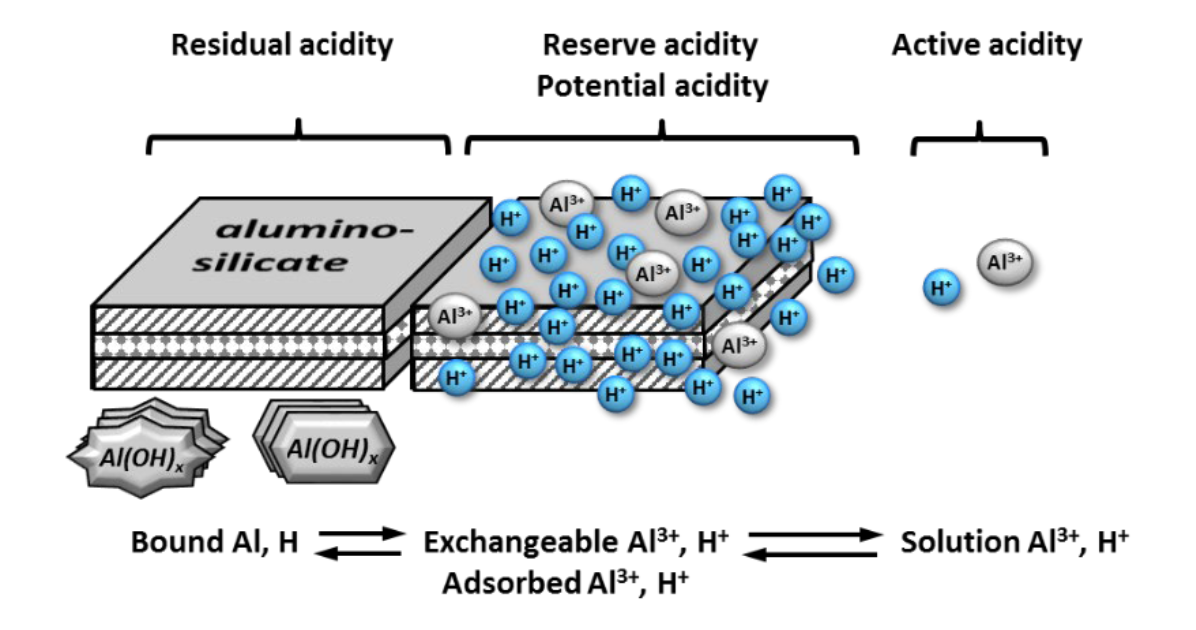
¶ A. Three “pools” or sources of soil acidity (see Fig. 1)
- Active soil acidity
- Hydrogen ion (H+) concentration of soil solution
- Measured in routine soil test using pH electrode
- Hydrogen ions in solution are in equilibrium with exchangeable hydrogen ions on exchange complex
- Very small part of total soil acidity
- Affects soil solution pH, so directly affects soil chemistry and reactions
- Hydrogen ion (H+) concentration of soil solution
- Reserve soil acidity
- Quantity of acidic cations (H+, Al3+) that occupy sites on exchange complex
- Reserve acidity exchanges with active acidity (hydrogen ions in solution)
- Significant component of buffering capacity
- Also referred to as "potential" acidity or “exchangeable” acidity
- Residual soil acidity
- Quantity of all bound aluminum and bound hydrogen ions contained in soil minerals or organic matter
- Ions are released by weathering and decomposition
- Is least available of soil acidity pools
- Also referred to as “non-exchangeable” acidity
¶ B. Buffering capacity
- Definition: ability of soil to maintain hydrogen ion concentrations in soil solution
- Buffering is ability to resist soil pH change in soil pH when H+ ions are added or removed
- Quantity of hydrogen (and aluminum) in each acidity pool is not permanently fixed
- Hydrogen and aluminum of one pool will replenish hydrogen and aluminum of another pool as these acid cations are removed
- Relative amounts change as hydrogen and aluminum move from one pool to another
- pH buffering: ability to replenish active acidity pool from reserve or residual acidity pools
- Pools of soil acidity react with each other to reestablish chemical equilibrium
- Reserve (exchangeable) acidity will buffer changes in active (solution) acidity over short-term
- Residual acidity will buffer changes in reserve and active acidity over long-term
- Well buffered soil
- Reserve acidity is able to quickly replenish H+ ions removed from soil solution and maintain level of active acidity
- When H+ ions are added to soil solution, reactions allow excess H+ ions to be adsorbed onto exchange sites
- Proportion of H+ ions in solution and on exchange complex remains constant; maintains equilibrium
- Soil pH changes very little in short term
- Poorly buffered soil
- Reserve acidity replenishes H+ ions slowly or is not able to completely replenish H+ ions that are removed from soil solution
- Few exchange sites are available to adsorb excess H+ ions; soil cannot maintain equilibrium
- H+ ion concentration (pH) of soil solution changes quickly
- Soil pH may slowly return to original level; may not return at all
- Long-term soil pH changes slowly in well-buffered soils
¶ C: Primary mechanisms of soil pH buffering
- Cation exchange capacity (CEC) affects buffering capacity
- H+ cation competes with other cations for exchange sites
- When soil pH is high (less acidic, more basic, low H+ concentration), more base cations will be on exchange complex
- Basic cations will be less susceptible to leaching
- When soil pH is lower (more acidic, less basic, higher H+ concentration), more H+ ions available to exchange with base cations
- H+ ions remove base cations from exchange sites
- Basic cations are released to soil solution
- Exchanged cations in solution can be taken up by plant or lost by leaching
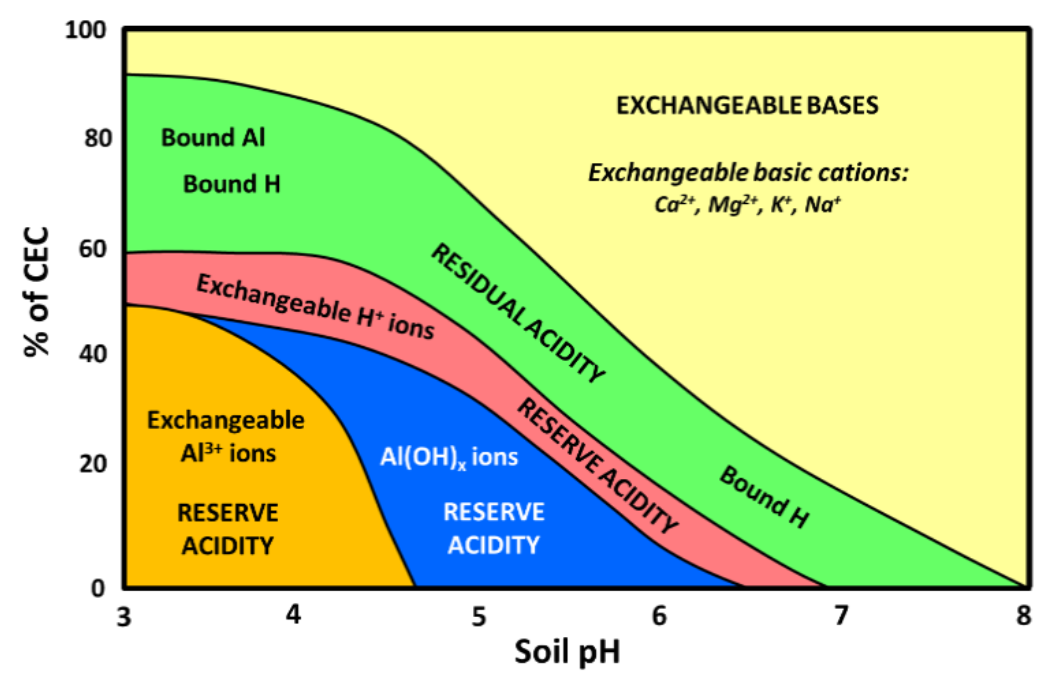
- Al3+ and hydroxyl aluminum ions occupy active exchange sites in acid mineral soils (pH 5.0 or lower)
- Many of these sites occupied by exchangeable bases (Ca2+, Mg2+, K+, Na+) at pH 6.0 (see Fig. 2)
- Carbonate reactions can neutralize natural or introduced acidity
- Bicarbonates and free carbonates are found in calcareous soils
- Limestone parent material may be original source of carbonates
- Carbonates have been leached from soil horizons in sub-humid or humid areas
- Carbonates may accumulate when annual precipitation exceeds evapotranspiration during growing season
- Bicarbonates may be applied through irrigation water
- Soil buffering by aluminum and hydroxyl-aluminum compounds
- Aluminum can function as either acid or base
- Adding acid or base shifts direction of aluminum reactions
- Acting as base, aluminum compounds can neutralize hydrogen ions (H+)
- Al(OH)3 + H+ ↔ Al(OH)2+ + H2O
- Al(OH)2+ + H+ ↔ AlOH2+ + H2O
- AlOH2+ + H+ ↔ Al3+ + H2O
- Al(OH)3 + 3H+ ↔ Al3+ + 3H2O
- Acting as acid, aluminum compounds can neutralize hydroxyl ions (OH-)
- Al3+ + OH- ↔ AlOH2+
- AlOH2+ + OH- ↔ Al(OH)2+
- Al(OH)2+ + OH- ↔ Al(OH)3
- Al(OH)3 + OH- ↔ Al(OH)4-
- Aluminum can function as either acid or base
- Reversible dissociation of H+ from pH dependent charge sites
- Includes organic matter surfaces, clay edges, oxide mineral surfaces
¶ Figure 1. Sources of Soil Acidity
¶ Figure 2. General Relationship Between Soil pH and Cations Held on Soil Colloid Exchange Surfaces
¶ D. Buffering capacity important for practical soil pH management
- Soil properties affect buffering capacity
- Fine-textured, high clay-content soils generally have higher CEC and greater buffering capacities than coarse-textured soils
- High organic matter soils generally have greater buffering capacities than low organic matter soils
- Clay mineralogy can affect buffering capacity
- Soils with mainly 1:1 clay minerals (Ultisols, Oxisols) generally less buffered than soils with primarily 2:1 clay minerals (Alfisols, Mollisols)
- Soil pH buffering capacity primarily affected by potential acidity
- Buffering capacity helps limit fluctuations in soil environment that could affect nutrient availability, microbial activity, and plant growth
- Reserve acidity buffers changes in active acidity
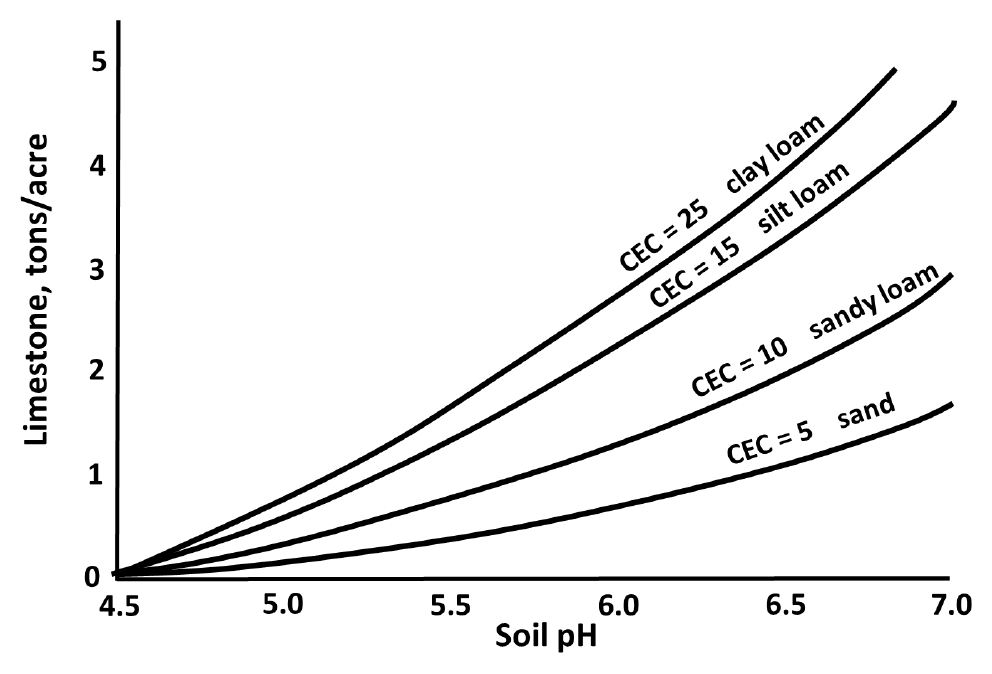
- Amount of soil amendment (lime, elemental sulfur, etc.) required to change pH to specified target value depends on components of soil buffering capacity (see Fig. 3)
- Soil with high buffering capacities requires larger amounts than soil with low buffering capacity
¶ Figure 3. Typical Relationship Between Soil Texture and Cation Exchange Capacity (mEq/100g) for Adjusting Lime Rate to Reach Target Soil pH