⇦ Back to Soil Fertility and Plant Nutrition Home
¶ No Single Optimum pH for Plant Growth
- Climate has impact on soil pH
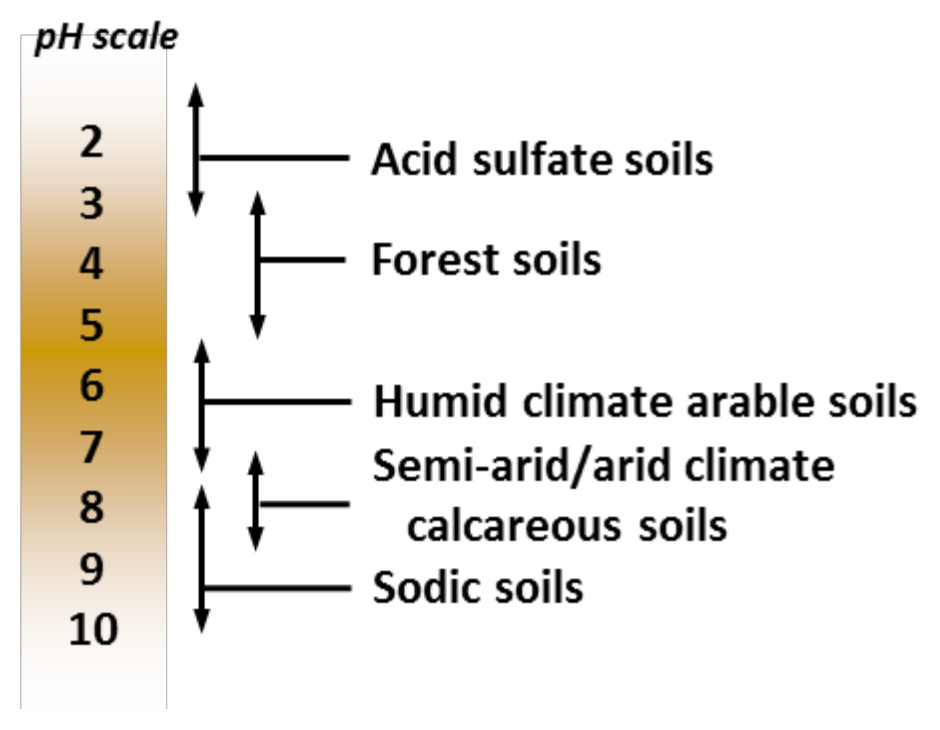
- Long-term precipitation patterns influence dominant vegetation and soil formation (see Figure 1)
- Higher precipitation increases weathering of soil minerals
- Releases acidic cations, like hydrogen and aluminum
- Increased vegetation growth removes increased amounts of basic cations, like calcium and magnesium
- “Ideal” pH range depends upon crop, rotation, and other specific conditions
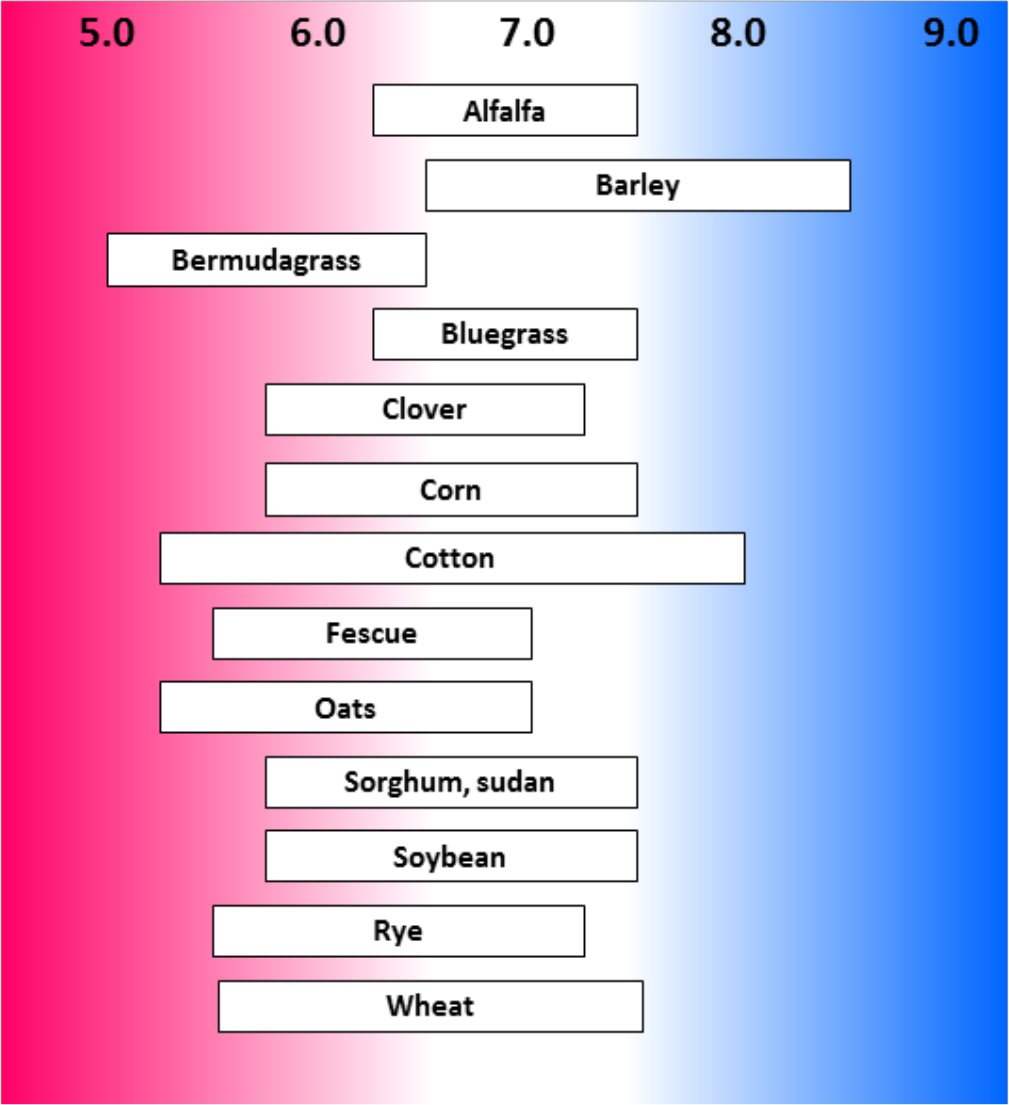
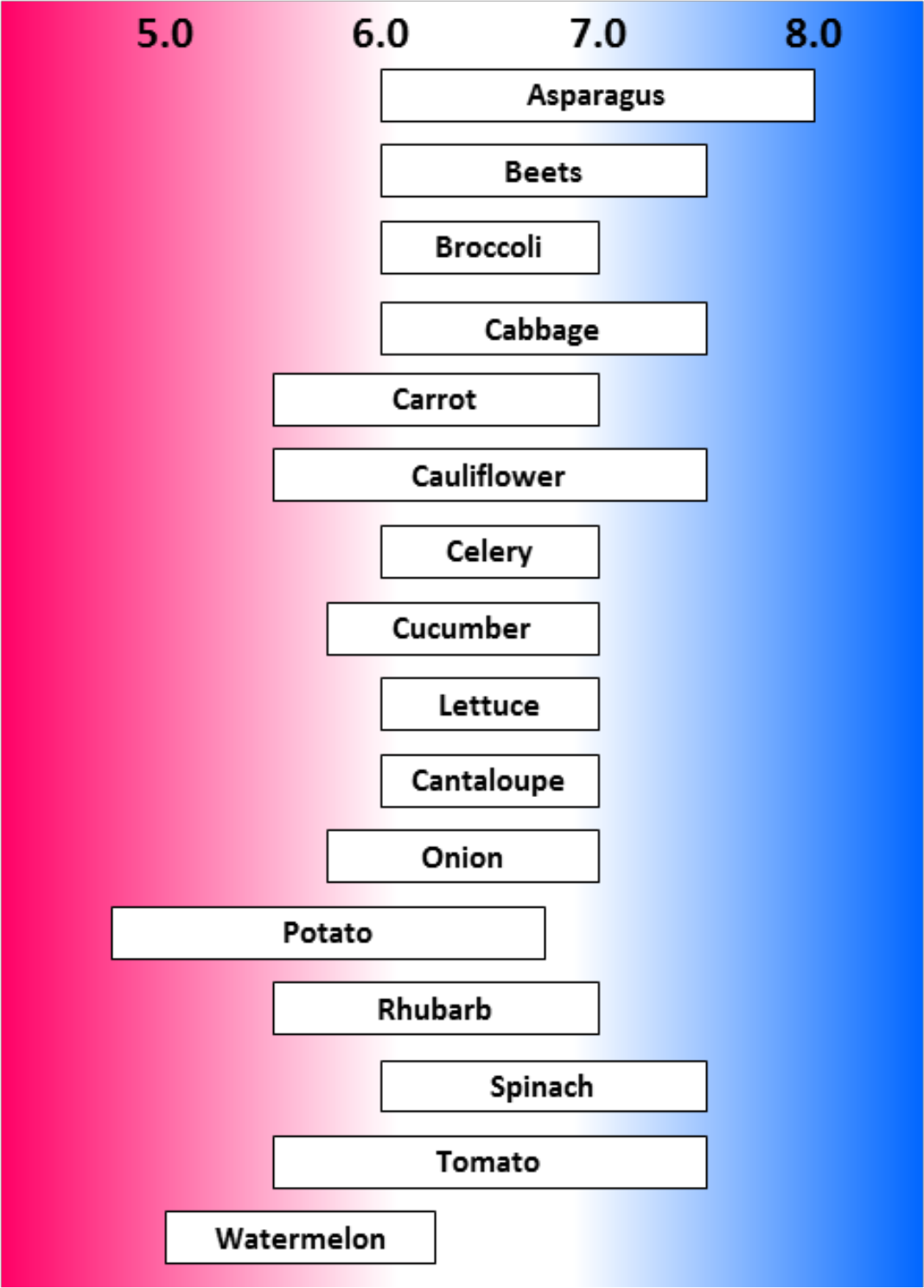
- Most crop plants grow very well in pH range of 6.0 to 7.0 (see Figures 2, 3, and 4)
- pH of most soils range between pH 4 .and pH 10.0
- Many plants do well from pH 5.5 to 7.0
- Legumes in rotation affect target pH range
- Most crop plants grow very well in pH range of 6.0 to 7.0 (see Figures 2, 3, and 4)
- Plants vary widely in their pH preferences
- Many legumes grow best in neutral to alkaline soils
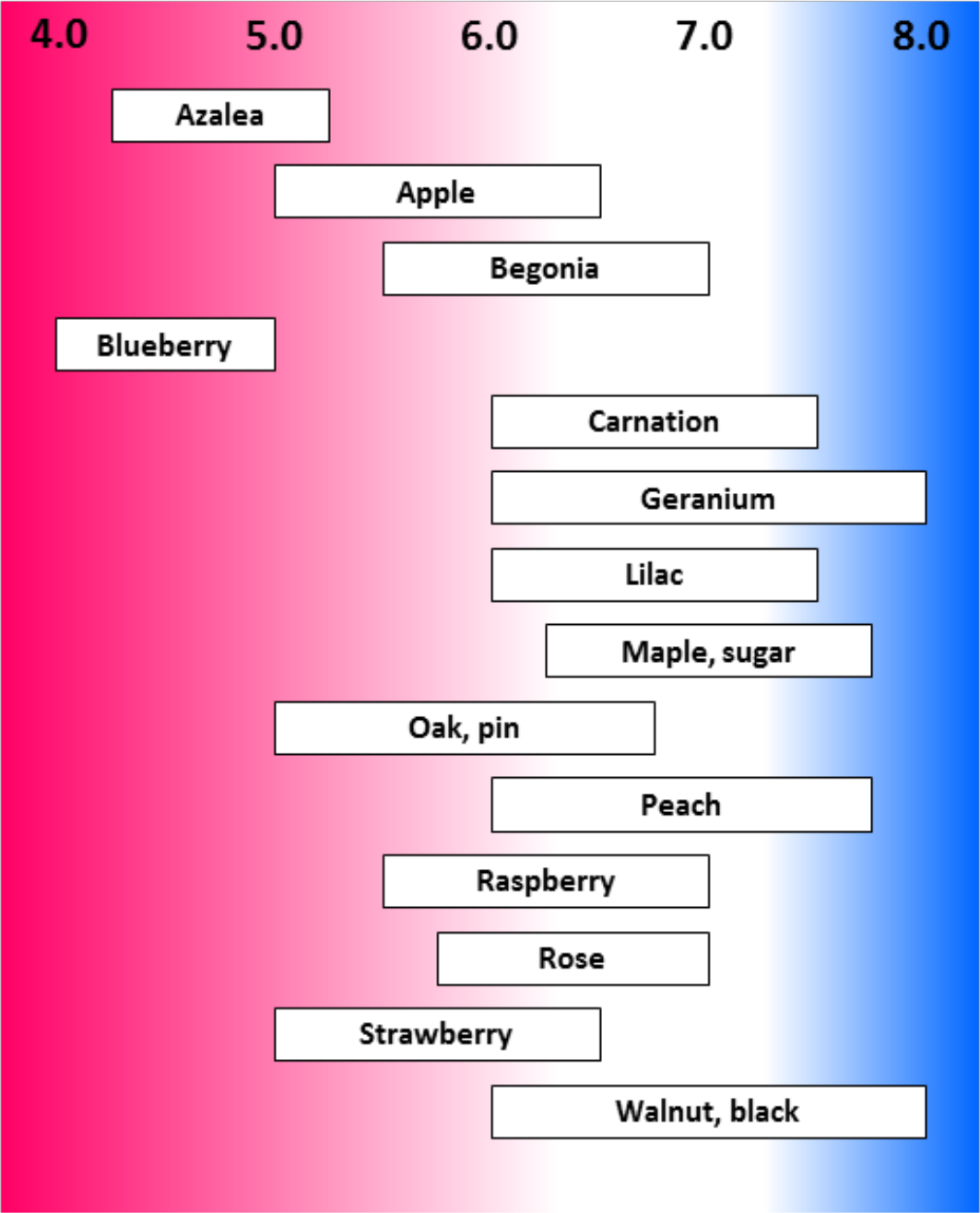
- Many forest trees grow well between pH 5.0 and 6.0
- Pine, aspen, and some spruce tolerate more acid conditions
- Conifers tend to intensify soil acidity
- Cranberries, blueberries, azaleas grow best in acid soils (pH less than 5.0)
¶ Figure 1. Typical Soil pH Range
¶ Figure 2. General Range of Desirable pH for some Agronomic Crops
¶ B. Soil pH indirectly affects crop growth by affecting other processes
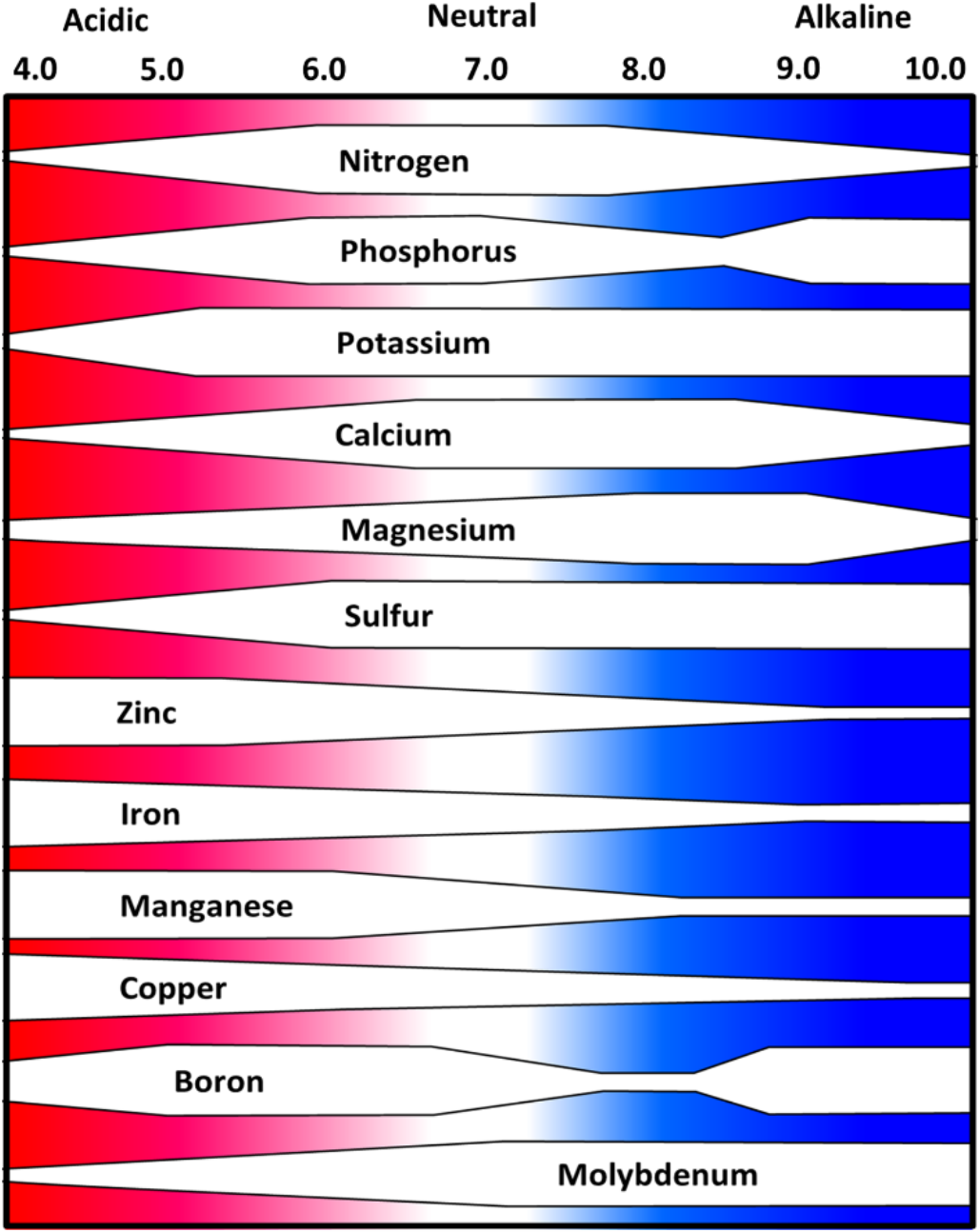
- Individual nutrients more or less available at high or low pH conditions
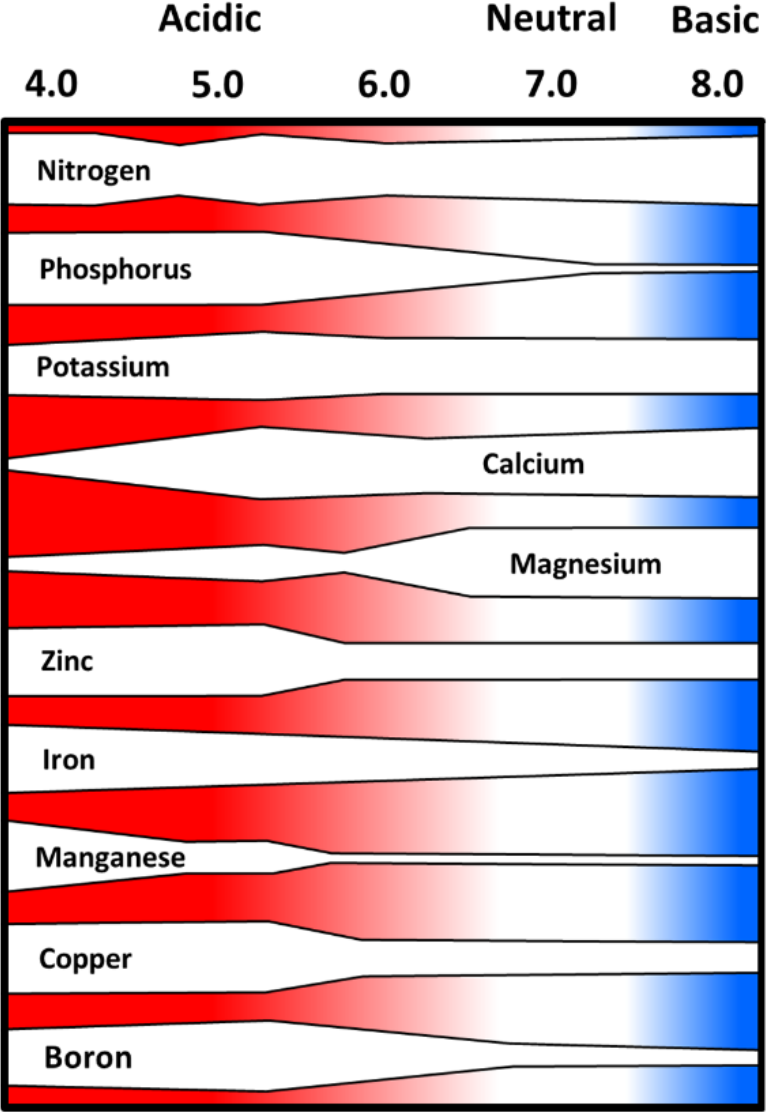
- pH range of 6.0 to 7.0 may be best overall for multiple nutrient availability in mineral soils (see Figure 5)
- Micronutrient metals tend to be less available when pH above 7.0
- Macronutrients and secondary macronutrients tend to be less available below 6.0
- Soilless media, organic soils have limited nutrient supply
- Are poorly buffered, so pH changes quickly affecting short-tern nutrient availability
- pH range of 6.0 to 7.0 may be best overall for multiple nutrient availability in mineral soils (see Figure 5)
- Acidic soil conditions
- Hydrogen ions (H+) can be directly toxic to roots, but rarely occurs
- H+ concentration not high enough until pH less than 4
- Increased solubility and availability of aluminum, manganese or iron can be toxic
- Increased solubility and availability of iron can be beneficial
- Rhododendrons and azaleas have high iron requirement; grow best in acid soils
- Reduced activity of some microbes may limit nitrification and other soil processes
- May improve plant health by inhibiting soil pathogens
- Potatoes grown at pH 5.3 or less to inhibit potato scab organism
- pH less than 7.0 reduces take-all disease in wheat
- Low pH may affect exchange capacity of certain clay minerals affecting nutrient availability
- Soils can develop some level of anion exchange capacity
- Basic cations (K+, Ca2+, Mg2+) tend to be more weakly bound, more prone to leaching
- Hydrogen ions (H+) can be directly toxic to roots, but rarely occurs
- Alkaline soil conditions
- Legumes often have high calcium or magnesium requirement
- Ca2+ and Mg2+ availability is greater in neutral to alkaline soils
- Reduced solubility of certain micronutrients can induce nutrient deficiencies
- Alkaline pH may be symptom of poor physical soil conditions
- Example: alkaline, calcareous subsoils exposed by erosion or land leveling ii. Low organic matter, poor soil structure, etc.
- Alkaline pH may be symptom of saline or sodic soils
- Excess salinity can reduce water uptake
- Excess sodium can limit air and water infiltration, soil permeability
- Elevated levels of chloride or sodium may be toxic
- May improve plant health by inhibiting soil pathogens
- Cauliflower and other crucifers grown at pH 7.0 or higher to inhibit germination of clubroot spores
- Legumes often have high calcium or magnesium requirement
¶ Figure 3. General Range of Desirable pH for some Horticultural Crops
¶ C. Deficiency/toxicity symptoms are affected by pH
- Aluminum toxicity is most important growth limiting factor in acid soil
- Problem when soil pH less than 5.5
- Soluble aluminum (Al3+) inhibits root growth
- Aluminum ion interferes with development of meristematic tissue (i.e. growing point) just behind root cap
- Short, stubby, “burned” roots are typical symptom
- May resemble nematode injury
- Damaged root system limits nutrient and water uptake
- Aboveground symptoms may appear as nutrient deficiency
- “Purpling” of leaves is common symptom
- Phosphorus uptake is limited by damaged roots
- Insoluble aluminum phosphates may precipitate inside leaf tissue
- Manganese toxicity
- Manganese minerals become highly soluble, perhaps toxic, when pH drops below 5.2
- Occurs frequently in tandem with aluminum toxicity
- Moist, organic, acidic soils are most susceptible to toxicity
- Typically occurs in poorly drained or flooded soils
- Iron deficiency chlorosis
- Occurs in high pH, calcareous soils
- Concentration of soluble iron sources declines as soil pH increases
- Fine carbonates neutralize acids secreted by plant roots that were meant to solubilize soil iron
- Plants differ in iron-uptake efficiency
- Wide range of ability to take up adequate iron with low levels of available iron in soil solution
- Are Fe-efficient vs. Fe-inefficient plants
- Occurs in high pH, calcareous soils
¶ D. Soil pH and soil organisms
- Microorganism growth is affected by pH
- Growth of many bacteria and actinomycetes inhibited with pH below 6
- Typically decompose cellulose and carbohydrates
- Fungi grow well across a wide range of soil pH
- Are prominent cellulose and lignin decomposers
- Dominant under acid conditions; less competition from bacteria and actinomycetes
- Growth of many bacteria and actinomycetes inhibited with pH below 6
- Nitrification greatly inhibited at pH below 5.5
- Nitrification = conversion of NH4+ to NO3-
- Requires population of Nitrosomonas and Nitrobacter species
- Nitrogen fixation restricted when pH drops below 6.0
- Survival of Rhizobia and other nitrogen-fixing species decrease in acid soils
- Inhibited by increased solubility of Al3+, Cu2+, Mn2+
- Nodulation process reduced by low pH
- Nodule numbers and weight are lower
- Low pH disrupts metabolic “communication” between host plant and bacteria
- Reduces rhizobia attachment to root hairs and colonization, so reduces nodule formation
- Survival of Rhizobia and other nitrogen-fixing species decrease in acid soils
- Earthworms do best when soil pH greater than 6.5
- Decomposition of plant residues and OM may be slow in acid conditions (pH below 5.5)
- Lignin is resistant to decomposition by bacteria; mainly decomposed by fungi
¶ Figure 4. General Range of Desirable pH for some Vegetable Crops
¶ Figure 5. Soil pH and Relative Nutrient Availability - Mineral Soils
¶ Figure 6. Soil pH and Relative Nutrient Availability - Soilless Media, Organic Soil