⇦ Back to Soil Fertility and Plant Nutrition Home
¶ A. pH Both Affects and Controls Ion Activity
- pH controls the chemistry and reactions of solutions
- pH is master variable; affects chemical forms of elements
- Solution chemistry is nutrient chemistry
- pH affects chemistry of solid surfaces in contact with solutions
- Affects mineral solubility, mineral weathering, mineral formation
- Soil pH affects exchange capacity
- Less weathered soils under neutral and alkaline conditions have CEC
- Highly weathered, tropical soils have CEC, but also have “anion exchange capacity (AEC)
- Exchange surfaces become positively charged
- Fertility management needed to prevent cation or anion nutrient deficiencies
¶ B. Soil pH affects Relative Nutrient Availability
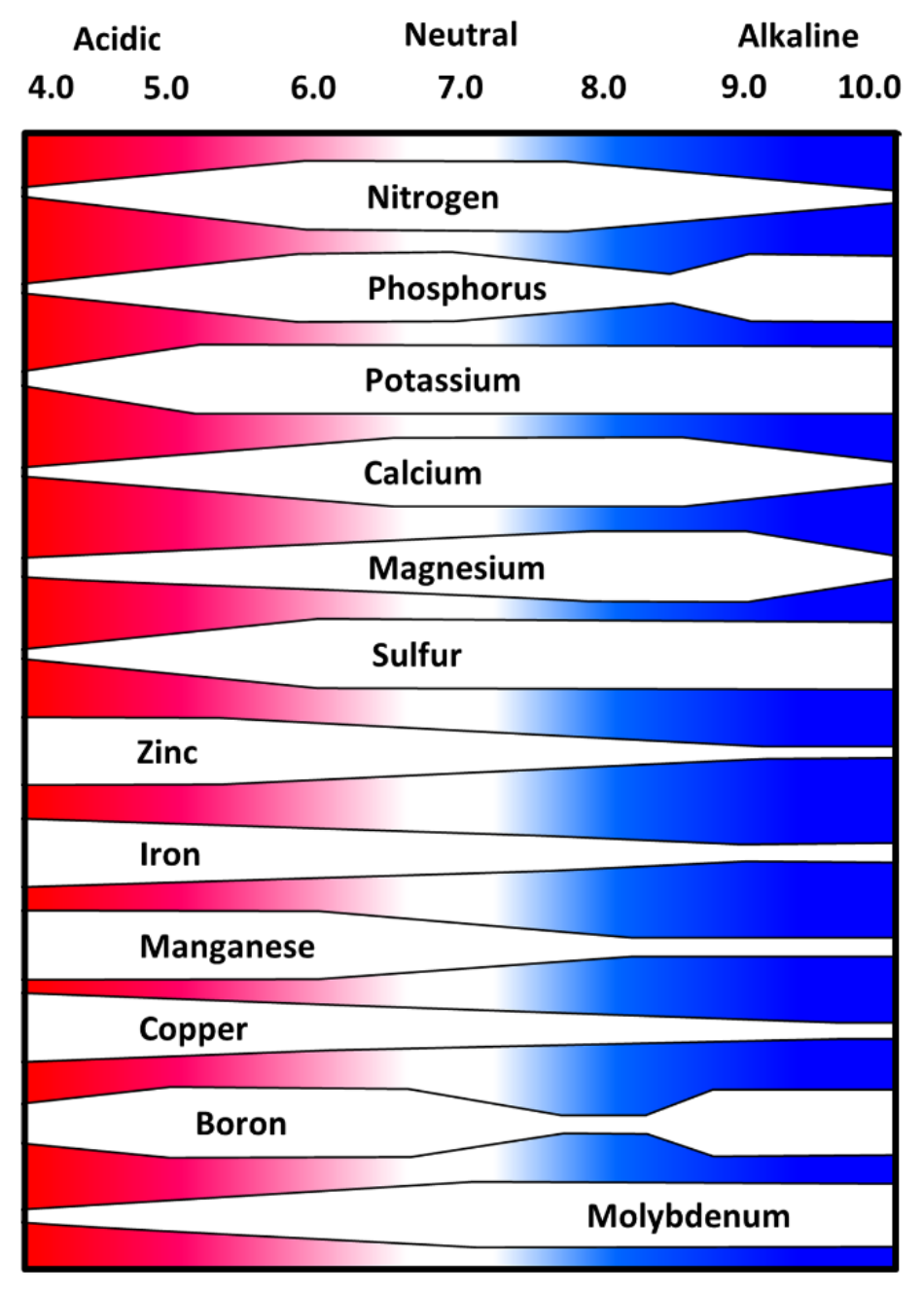
- Figure 1: Soil pH and relative nutrient availability
- Width of white areas in Figure 1 represents change in relative plant availability of essential plant nutrients at increasing pH levels
- Relative “plant availability” is considered to be overall ability to solubilize nutrient ions at specified pH level
- Macronutrients (N, P, K)
- Nitrogen (N) and potassium (K) generally more available within pH range of 6.5 to 8.0
- Generally less available as pH decreases below 6
- Basic cations (K) more weakly bound at low pH, prone to leaching
- Phosphorus generally most available within pH range of 6.0 to 7.0
- Nitrogen (N) and potassium (K) generally more available within pH range of 6.5 to 8.0
- Secondary macronutrients (Ca, Mg, S)
- Generally more available within pH range of 6.5 to 8.0
- Generally less available as pH decreases below 6
- Basic cations (Ca2+, Mg2+) more weakly bound at low pH, prone to leaching
- Micronutrients (Zn, Fe, Mn, Cu, B, Mo)
- Generally more plant available within a pH range of 5 to 6
- Deficiencies may occur at pH greater than 7
- e.g. iron deficiency chlorosis
- Boron less available as pH increases above 7
- Deficiencies may occur at pH greater than 7
- Plant availability often highest under acid conditions
- Toxicity may occur at very low pH (pH <5.5)
- e.g. manganese toxicity
- Molybdenum less available at low pH
- Toxicity may occur at very low pH (pH <5.5)
- Generally more plant available within a pH range of 5 to 6
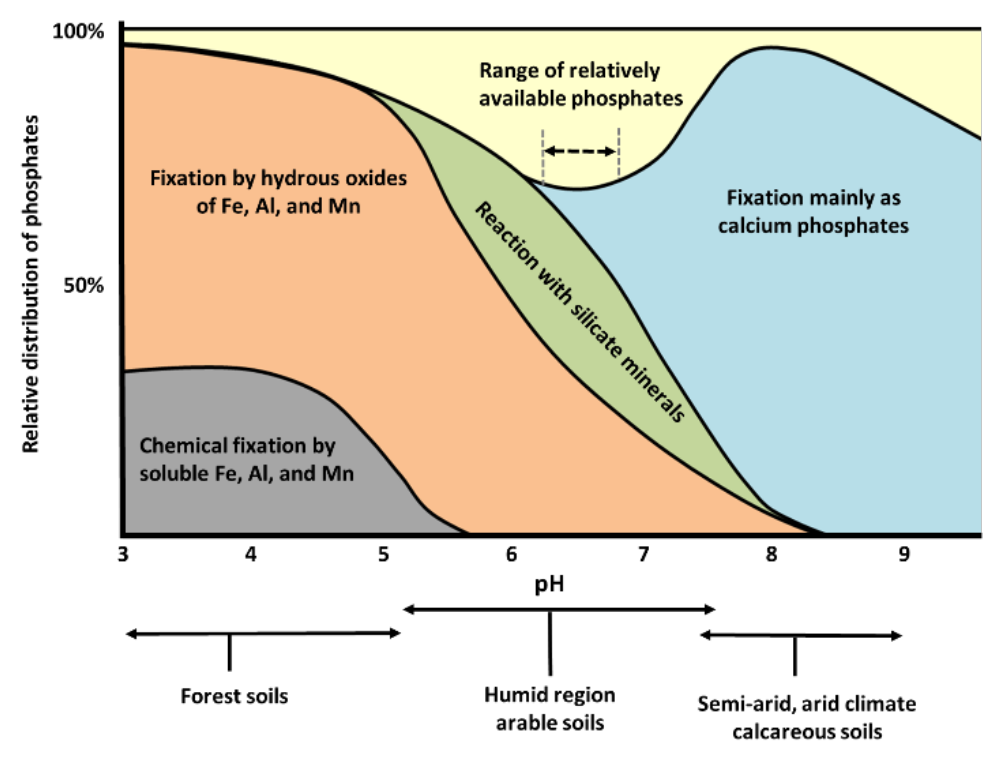
¶ Figure 1. Soil pH and Relative Nutrient Availability - Mineral Soils
¶ C. Example: pH effect on soil phosphorus
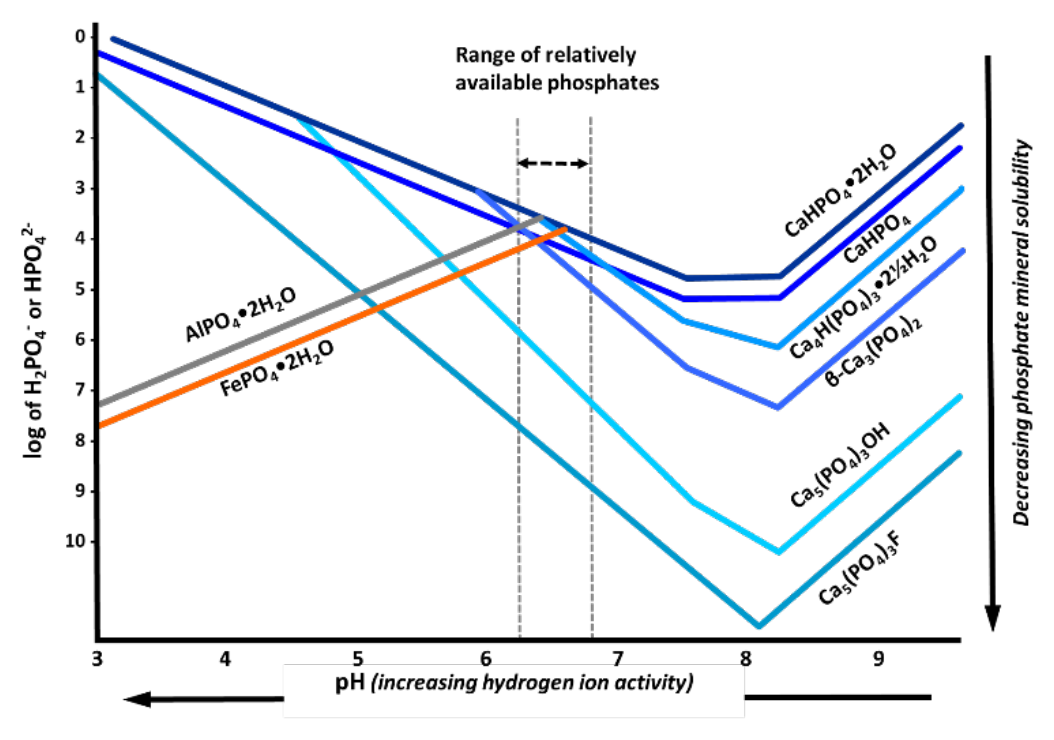
- Mineral abundance in solution changes with pH
- Iron and aluminum are most abundant soluble cations in acidic and highly acidic soils
- Leads to precipitation of aluminum phosphate and iron phosphate minerals
- Phosphate ions become adsorbed and incorporated into iron and aluminum oxides
- Concentrations of soluble iron and aluminum decrease as pH increases
- Iron and aluminum orthophosphates are less soluble - more stable - in highly acid soils (Fig. 2a)
- Iron and aluminum phosphate solubility increases as pH increases
- At about pH 6.5, overall mix of calcium, iron, and aluminum phosphates is optimized; relative availability is typically greatest
- Iron and aluminum are most abundant soluble cations in acidic and highly acidic soils
- Solubility of all calcium phosphates declines with increasing pH
- Calcium phosphates are highly soluble (less stable) in highly acid soils
- Phosphorus is fixed by increasingly complex and stable calcium minerals
- DCPD is most soluble; fluoroapatite is least soluble
- At solution pH of about 7.8 and above, solubility of calcium phosphates begins to increase
- Are typically calcareous soils
- High pH results in carbonates precipitation
- Calcium ion (Ca2+) concentration is depressed in calcareous soils
- Form less stable (more soluble) calcium minerals
- Are typically calcareous soils
- At solution pH range of 6.0 to 6.5 several phosphate minerals can coexist
- Variscite, strengite, CDPD, DCP, OCP, and β-TCP
- All six minerals maintain concentration of about 10-3.25 M H2PO4-
- Is range of optimum plant availability
- Figure 2a, Solubility of orthophosphate minerals
- X axis: solution pH
- Acidity decreases from left to right
- Y axis: log of orthophosphate ion concentration in soil solution
- Ion activity (i.e., solubility) decreases from top to bottom
- Each one unit change is 10-fold change in mineral solubility
- Plotted lines: relative solubilities of eight most abundant orthophosphate minerals (see Table 1 for names and chemical formulas)
- X axis: solution pH
- Figure 2b, Relative distribution of added phosphates
- X axis: soil pH (acidity decreases from left to right)
- Y axis: approximate percentage distributions
- Plotted data: mechanisms that potentially affect phosphorus fixation and relative plant availability
- Below chart: typical pH range of regional soil types and general climate
¶ Table 1. Common Soil Phosphorus Minerals
| Acid Soils | Formula |
| Variscite | AlPO4 • 2H2O |
| Strengite | FePO4 • 2H2O |
| Neutral and Calcareous Soils | Formula |
| Dicalcium phosphate dehydrate (DCPD) | CaHPO4 • 2H2O |
| Dicalcium phosphate (DCP) | Ca5HPO4 |
| Octacalcium phosphate (OCP) | Ca4H(PO4)3•2½H2O |
| β-ttricalcium phosphate (βTCP) | β-Ca3(PO4)2 |
| Hydroxyapatite (HOA) | Ca5(PO4)3OH |
| Fluoroapatite (FAP) | Ca5(PO4)3F |
¶ Figure 2a. Solubility of Orthophosphate Minerals at Different Soil pH Values
¶ Figure 2b. Relative Distribution of Phosphates at Different Soil pH Values