⇦ Back to Soil Fertility and Plant Nutrition Home
¶ A. Solution Chemistry is Soil Chemistry
- Soil solution
- Definition: aqueous phase of soil and its solutes
- Aqueous: of or containing water
- Solute: substance dissolved in another substance, known as a solvent (i.e. water)
- Solvent is major fraction of mixture
- Any solution takes on characteristics of solvent
- Components of soil solution
- Solvent = water
- Solutes = minerals, dissolved gases, and organic matter
- Aqueous phase = water held on soil particle surfaces and in voids (spaces) between soil particles
- Definition: aqueous phase of soil and its solutes
- Soil pH
- Is not pH of minerals or of solid organic materials making up bulk soil
- Is pH of soil solution
- Chemistry of soil acidity: major concepts
- Hydration and ionic activity
- Hydrogen and aluminum are major sources of soil acidity
- Acidity is measured as hydrogen ion (H+) concentration, but aluminum ion (Al3+) activity is primary source of H+
¶ B. Hydration
- Hydration = shell of water or "solvation sphere" bound to ions in solution
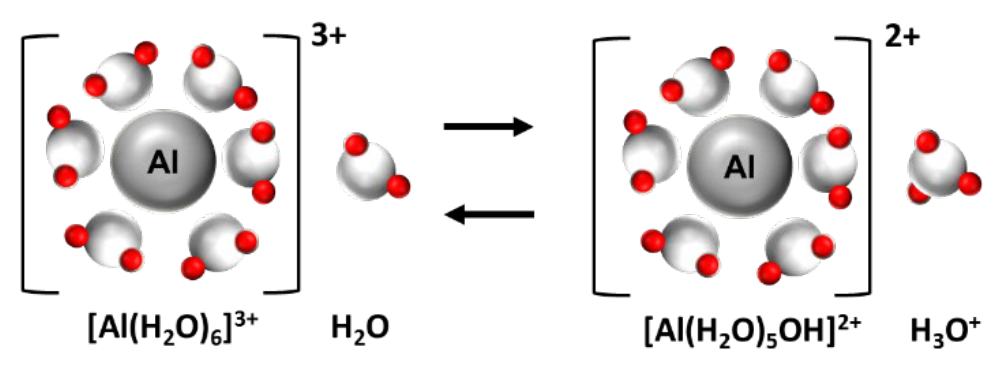
- Example: Al3+ in solution actually exists as a hydrated complex; is actually [Al(H2O)6]3+ (see Fig. 1)
- Hydrogen ions are always hydrated
- pH actually measures the activity of H3O+ rather than H+
- Activity or concentration
- Ionic activity is the "effective concentration" of an ion
- Takes into account the effects of all other ions in solution on reactivity of that ion
¶ Figure 1. Acidic Nature of Hydrated Aluminum Ion in Water Solution
¶ Table 1. Mechanisms That Control Soil pH |
|
| Soil pH Range | Major Mechanisms |
| 2.0 to 4.0 | Oxidation of reduced sulfur minerals, pyrite (FeS2) |
| 4.0 to 5.5 | Exchangeable aluminum and hydrogen (Al3+, Al(OH)x, H+) |
| 5.5 to 6.8 | Exchangeable hydrogen (H+) |
| 6.8 to 7.2 | Dissolved carbon dioxide (CO2) |
| 7.5 to 8.5 | Dissolution of carbonates (CaCO3) |
| 8.5 to 10.5 | Exchangeable sodium Dissolution of sodium carbonate (NaHCO3) |
¶ C. Sources of Soil Acidity: Hydrogen
- Soil organic matter sources
- Carboxylic acid and phenolic groups
- Behave as weak acids releasing H+
- Example: R-COOH ↔ R-COO- + H+
- “R” group = any group in which carbon or hydrogen atom is attached to rest of molecule
- Generate pH dependent charge as well as acidity
- Importance depends upon organic matter content of soil
- Clays and oxide minerals
- Generate pH dependent charge reactions
- Dissociation of H+ from clay edges and from aluminum and iron oxide surfaces
- Example: clay_edge-Al, Si-OHH+ ↔ clay_edge-Al, Si-OH + H+ ↔ clay_edge-Al, Si-O- + H+
- Exchangeable hydrogen
- H+ is an exchangeable cation
- Exchange with Ca2+ or other cations can increase solution acidity
- Exchangeable H+ is generally low
- Exchangeable acidity is predominantly due to aluminum
¶ D. Sources of Soil Acidity: Aluminum
- Adsorbed aluminum ion (Al3+) is exchanged and released to solution
- Hydrolysis of aluminum ions produces H+
- Al3+ + H2O ↔ AlOH2+ + H+
- Series of hydrolysis reactions can occur, depending on pH
- AlOH2+ + H2O ↔ Al(OH)2+ + H+
- Al(OH)2+ + H2O ↔ Al(OH)3 + H+
- Al(OH)3 + H2O ↔ Al(OH)4- + H+
- Aluminum hydroxy ions are adsorbed and act as exchangeable cations
- AlOH2+ and Al(OH)2+ act as exchangeable cations
- Little free aluminum, (Al3+) above pH 5
- Can also form complex hydroxy-aluminum polymers
- Are nonexchangeable
- Have high positive charge, held very strongly on exchange surfaces
- Little soluble aluminum above pH 7
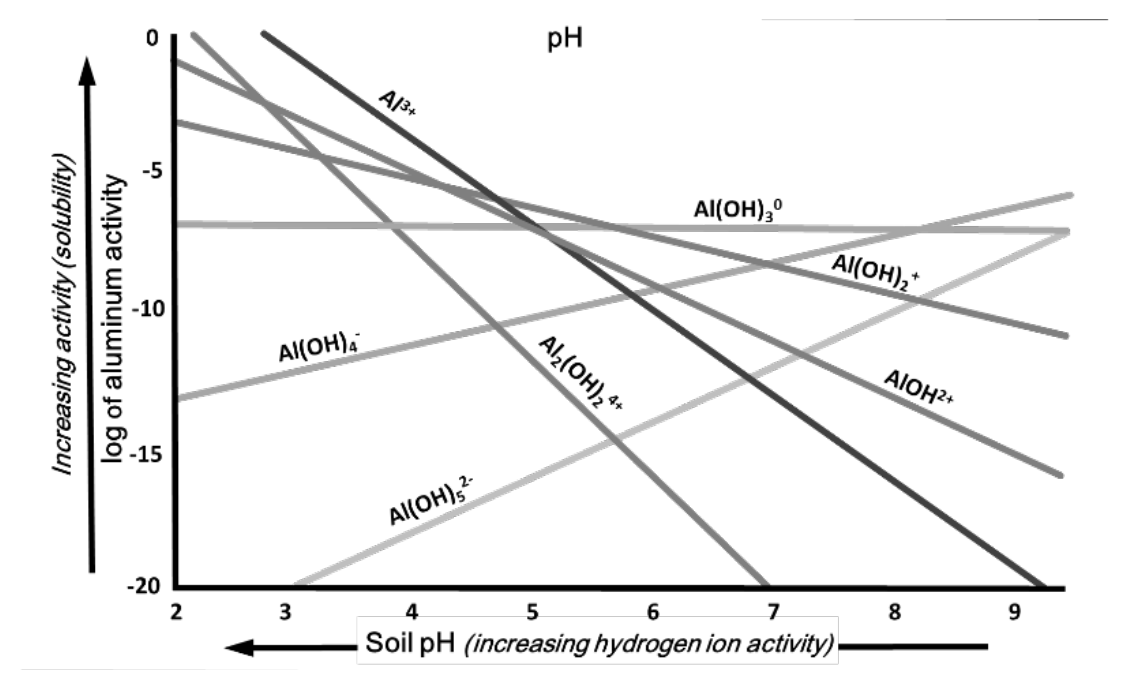
- Al(OH)3 (gibbsite) is insoluble across wide range of pH (see Fig. 2)
- At pH 5 to 5.5, soluble aluminum ion (Al3+) ion activity becomes lower than gibbsite
- At pH 8, other aluminum minerals are less soluble than gibbsite
¶ Figure 2. Activité of Soluble Aluminum and Hydrolyzed Compound Activities at Different pH
¶ E. Natural Mechanisms Affecting Soil Acidity (see Table 1)
- Parent material
- pH of rocks from which soils formed can vary greatly
- Soils formed from sandstone, shale, granite, silica (rhyolite) more acidic than those formed from limestone
- Sandy soils with low buffering capacities are more acidic
- Removal/replacement of basic cations
- Basic cations (Ca2+, Mg2+, K+, Na+) removed by crop uptake
- Higher yields increase uptake and removal over time
- Legumes remove more Ca2+, Mg2+, than corn or sorghum
- Basic cations weathered from soil minerals in surface, then leached into subsoil or deeper into profile
- Occurs in climates where precipitation exceeds evapotranspiration
- Basic cations subsequently replaced by acidic cations (H+, Al3+)
- Basic cations (Ca2+, Mg2+, K+, Na+) removed by crop uptake
- Precipitation
- pH of natural rain is acidic; pH = 5.6 to 5.7
- Caused by CO2
- Forms carbonic acid
- H2O + CO2 ↔ H2CO3
- H2CO3 ↔ HCO3- + H+
- Carbonic acid dissolves carbonates, releasing basic cations (Ca2+, Mg2+, K+, Na+)
- Example: H+ + CaCO3 → HCO3- + Ca2+
- Basic cations can be leached when amount of stored moisture plus rainfall approximately equals or exceeds amount of evapotranspiration
- Natural deposition
- Volcanic activity deposits acidic H2SO4
- Lightning deposits acidic HNO3
- pH of natural rain is acidic; pH = 5.6 to 5.7
- Decomposition of organic residues
- Amount of residue and decomposition rate affected by climate and vegetation type
- Soil pH tends to be regional (see Table 2)
- Annual temperature: tropical vs. temperate
- Annual precipitation: humid vs. sub-humid vs. semi-arid
- Dominant vegetation: forest vs. grassland
- Prairie grass soils generally less acidic than forested soils
- Coniferous tree residue more acidic than deciduous tree residue
- Decomposition produces organic acids, like fulvic acid or humic acid
- CO2 produced from microbial respiration during decomposition, forms carbonic acid (H2CO3)
- Iron hydrolysis
- Example: Fe3+ + H2O ↔ FeOH2+ + H+
- Not a major factor in soil acidity until all aluminum has reacted
¶ F. Introduced sources of soil acidity
- Fertilizers
- Primarily ammonium (NH4-N) sources
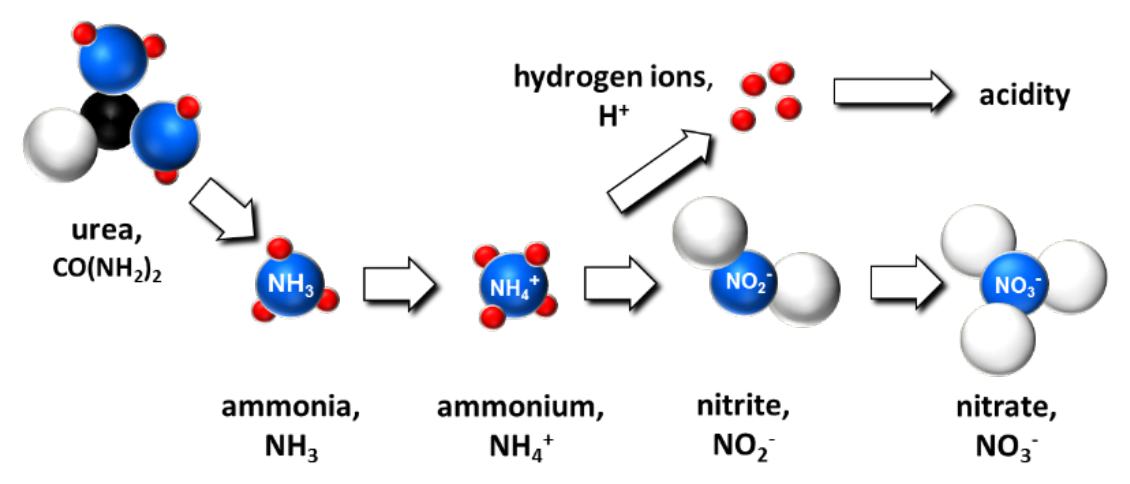
- Produce H+ during conversion (nitrification) of NH4+ to NO3- (see Fig. 3)
- Includes ammonium forming nitrogen sources, like urea, anhydrous ammonia
- Higher nitrogen rates, increased acidity
- Typically requires 1.8 to 5.4 lb. CaCO3 to neutralize acidity of each 1 lb. of ammonium-type fertilizer applied (see Table 3)
- Ammoniated phosphate fertilizers may cause highly localized zones of acidity
- Primarily ammonium (NH4-N) sources
- Mining sites
- Pyrite (iron disulfide, FeS2) is important sulfide found in waste rock
- Forms sulfuric acid when exposed to water and oxygen
- Pyrite oxidation: 2FeS2 (solid) + 7O2 + 2H2O → 2FeSO4 + 2H2SO4
- Oxidation of ferrous iron to ferric iron: 2Fe2+ + ½O2 + 2H+ → 2Fe3+ + H2O
- Iron hydrolysis: Fe3+ + 3H2O → Fe(OH)3 + 3H+
- 4FeS2 (solid) + 15O2 + 14H2O ↔ 16H+ + 8SO4- + 4Fe(OH)3 (solid)
- Cause of environmental problems from acid drainage
- Use of fossil fuels
- Gaseous nitrous oxide (NO) and/or sulfur dioxide (SO2) into atmosphere by burning of coal and petroleum fuels
- Gases react with oxygen to form HNO3 and H2SO4
- Contribute to acid rain
- Acid rain can have pH of 4.5 or lower
- pH of natural rain, 5.6 to 5.7
- Air quality regulations have resulted in reduced emissions
¶ G. Sources of soil alkalinity
- Base-forming cations
- From exchangeable Ca2+, Mg2+, K+, Na+
- Example: clay-Ca2+ + H2O ↔ clay-2H+ + Ca2+ + 2OH-
- Carbonates and bicarbonates
- Free carbonates in calcareous soil can neutralize natural or introduced acidity
- Soil carbonates accumulate in dry climates
- Annual evapotranspiration exceeds annual precipitation during growing season
- Little or no leaching of base cations
- Carbonates serve as source of hydroxyl ions (OH-)
- Example: NaHCO3 + H2O ↔ Na+ + H2CO3 + OH-
- Bicarbonates in irrigation water
- Each 1 mg/L HCO3- equivalent to 0.3 lb CaCO3/acre-inch
¶ Figure 3. Nitrification Yields Hydrogen Ions and Acidity
¶ Table 2. Acidity Generated From Common Fertilizers |
|
| Nitrogen source | Lb CaCO3/Lb N Needed to Neutralize Acidity |
| Anhydrous ammonia (82-0-0) | 1.8 |
| Urea (45-0-0) | 1.8 |
| Ammonium sulfate (21-0-0) | 5.4 |
| UAN solution (28-0-0, 32-0-0) | 1.8 |
| Monoammonium phosphate (18-46-0) | 5.4 |
| Diammonium phosphate (18-46-0) | 3.6 |