⇦ Back to Soil Fertility and Plant Nutrition Home
¶ Introduction
Plant tissue analysis is the method we use to assess plant nutrient uptake. Good plant tissue sampling and handling procedures are essential to interpret the laboratory results properly. Ratings and interpretations of the lab results have been developed for specified plant parts sampled at particular growth stages or time for a certain plant species. If plants are not sampled correctly, the interpretations may be meaningless.
Monitoring is used to observe and track the nutrient status of healthy plants during the growing season. The objectives are to fine-tune the fertility management or to detect “hidden hunger” and correct it, as necessary. Monitoring samples are taken from a uniform, consistent area on a schedule, often collected weekly or biweekly.
Problem-solving or diagnostic samples are taken to confirm whether visible symptoms are due to nutrient deficiency or to elemental toxicity.
Following are general guidelines for collecting plant samples. Refer to individual Crop Files for specific sampling guidelines and interpretation guidelines.
¶ When to Sample
The best time to collect samples is between midmorning and mid-afternoon, though exact timing is not critical. Samples should be taken at the same time of day, when feasible, to maintain consistency in the sequence.
Nutrient levels may be affected by time of day, drought, cloud cover, or other factors. As an example, nitrate and potassium move readily within the plant with water, so levels can change rapidly depending on environmental conditions.
Plant tissue samples can be collected during four time-frames during the growing season:
- Emergence and early vegetative stages (during emergence and the beginning of rapid growth)
- Late vegetative stages (during rapid growth and right at the beginning of reproductive stages),
- Early reproductive stages (during flowering and early seed set or fruiting), and #4. late reproductive stages to maturity (during seed or fruit ripening to physiological maturity and senescence).
¶ Monitoring Healthy Plants
Time-frames #1 and #2: Samples are collected weekly, biweekly, or monthly during the growth stages with increased nutrient demand. The results are used to adjust nutrient application plans during the current season.
The proper timing depends on the specific crop or plant. For example: cotton is sampled from match-head square to mid-bloom, corn is sampled from early growth to tasseling, and orchards are sampled during mid-season.
collected during the later part of the growing season. These are a “look-over-the-shoulder” samples used to evaluate the past seasons’ fertility management and/or to plan for next season.
¶ Problem Solving, In-Season
Diagnostic samples can be collected anytime during the growing season when symptoms or problems are obvious. Samples should be collected in pairs (as described later) when using tissue analysis as part of the problem-solving process.
¶ Where to Sample
Take samples from a uniform area, whatever the intended use. If the field has variations in soil type, topography or field history, take multiple samples so that each unique area is represented by its own unique sample. All tissue within a single sample should be from the same cultivar or variety to avoid genetic differences in nutrient uptake and assimilation.
¶ Monitoring Samples
It is very important to collect plant samples from the same area each time the field is sampled.
One strategy is to flag 12 to 15 specific locations within the field. The locations may be random or can be laid out in a systematic pattern (see Figure 1). Take plant samples near each flagged location, combine them, and make a composite sample for shipping to the laboratory.
¶ Figure 1.
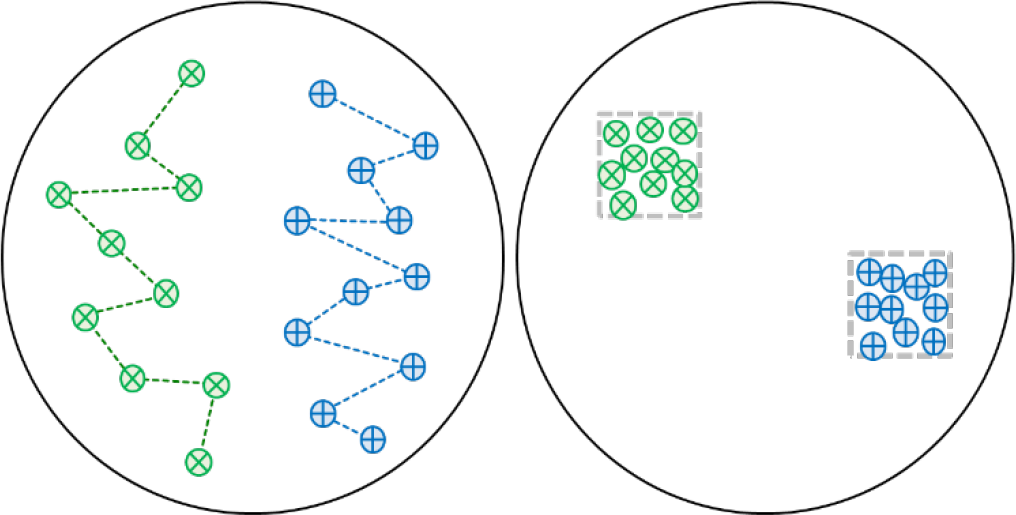
Another monitoring strategy is to flag a small, “benchmark” area that represents the entire field (see Figure 1). Subsamples from within the area are composited to form a single sample.
¶ Diagnostic Samples
The results of a single plant sample taken once during the season can be misleading. Critical nutrient ranges are developed over time from a large, diverse population of plants and can vary greatly.
Take paired samples, one from the “good” area, one from the “bad” area, for best results. Avoid “dead” areas (see Figure 2). Comparing results from the two samples can help pinpoint the limiting nutrient. Paired samples taken under identical conditions help factor out the influence of field stresses, different varieties, etc.
¶ Figure 2.
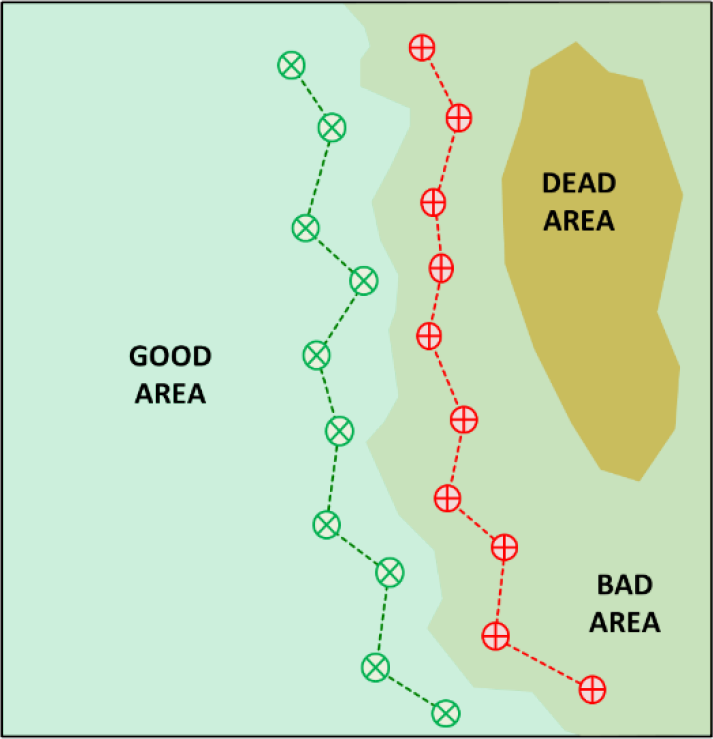
Always take matching soil samples from the root zones of both “good” and “bad” plants for the complete evaluation. Paired soil samples will help verify if the problem is a true nutrient deficiency or if nutrient uptake is being disrupted. Non-nutrient causes may include adverse soil pH, excess salinity, drought or water-logging, root injury, soil compaction, disease, or others.
¶ What to Sample
The appropriate plant part depends on the crop, the growth stage, and purpose of sampling. The proper parts include:
- whole plants,
- recently matured, fully expanded leaves (leaf blade alone or leaf blade with petiole), and
- petioles (leaf stalks) from recently matured leaves.
Sampling guidelines are given in the individual Crop Files, but some general plant sampling guidelines are:
- Young plants (4 to 6 inches tall): Sample the entire plant, cutting it off about one-half to one inch above the soil line. Do not include roots or soil with the plant tissue sample
- Small grains or grasses: Sample whole stems or tillers, cutting them off about one-half to one inch above the soil line. Do not include roots or soil with the sample
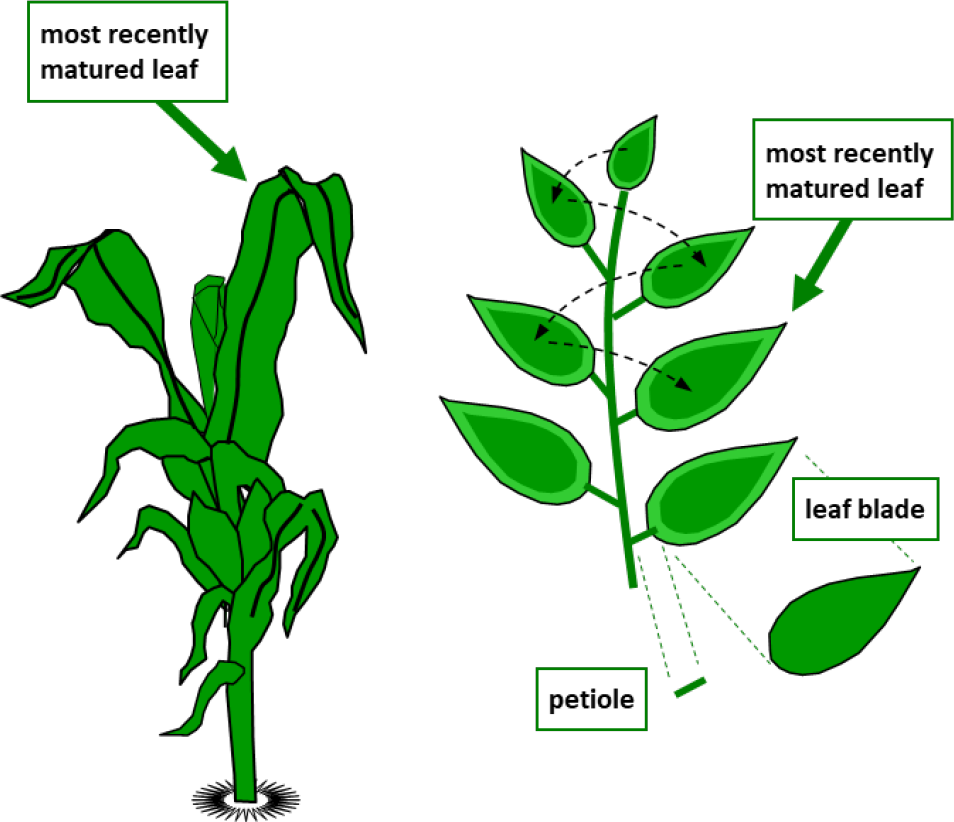
- Growing plants: A general rule is to sample the “MRML” or “most recently mature leaf” (see Figure 3). This is a leaf that is fully emerged, full-sized, and fully expanded. The MRML is often the 3rd to 5th leaf below the terminal leaf, growing point or whorl. The leaf is neither dull from age nor shiny green from immaturity.
The MRML is a compound leaf for some crops. For example, the MRML on soybean or strawberry is a trifoliate compound leaf (three leaflets comprising one leaf)
¶ Figure 3.
¶ Monitoring Healthy Plants
Take samples during recommended growth stages (see individual Crop Files). Do not take samples from plants that have obviously been stressed or injured by causes other than nutrients, including chemical, mechanical, or weather stresses.
¶ How Much to Sample
A good sample contains enough tissue to represent the entire area being sampled, but how much is that? A representative plant tissue sample must provide an average of nutritional status for an entire field, not just one or two plants. Therefore, the larger the area sampled, the larger the sample size needed.
A final sample volume between about a pint and a quart is recommended for complete analysis
- For most crops, a composite sample of leaves or parts from 15 to 30 plants is best
- For crops with small leaves, a composite sample of 30 to 50 leaves from those 15 to 30 plants is necessary
- For crops requiring petiole analysis, collect at least 20 to 50 petioles
- For very large-leafed plants, six to eight leaves may be adequate
General guidelines for judging final sample volume are:
- A bundle of loosely packed tissue (leaves or very small plants) that can be enclosed by touching the tips of all four fingers and thumbs, about softball size (Figure 4)
- A bundle of grass stems or small grain tillers that can be encircled by your thumb and index finger (Figure 5)
¶ Figure 4.

¶ Figure 5.

¶ How to Package and Ship a Sample
Brush off or shake off as much dust or dirt as possible. Dust and dirt have high iron and aluminum content that can inflate the tissue analysis results.
Do not wash or rinse the tissue sample. Soluble nutrients, like potassium, can be leached from plant tissue by excess washing. We will wash the sample - as needed - after it is received by the laboratory.
Pack the sample loosely in a paper bag or envelope. This allows the sample to begin drying during shipment.
Do not use plastic bags or cloth bags. Plastic bags trap moisture and may lead to molding and sample deterioration.
If there is more than a one-day delay between sampling and shipping, store the samples in a refrigerator, but do not freeze them.
Ship the sample by the fastest means feasible to avoid deterioration. Commercial package services are preferred. We have found the USPS Priority Mail delivery times to be inconsistent.
¶ Figure 6.

¶ References
Cleveland, B., M. McGinnis, and C. Stokes. 2008. Sampling for Plant Analysis. Agronomic Sampling Folder No. 5. North Carolina Department of Agriculture and Consumer Services - Agronomic Division, Raleigh NC
Peters, J.B. 2007. Getting full value from tissue testing. Proceedings, Wisconsin Fertilizer, Aglime & Pest Management Conference. Madison, WI. pg. 140-146.