⇦ Back to Soil Fertility and Plant Nutrition Home
¶ Introduction
This Crop File discusses use of the buffer pH method to make agricultural lime rate recommendations. It also discusses the rationale ServiTech Laboratories used for adopting the Sikora-1 and Sikora-2 methods.
¶ Buffer pH
Background: Adjusting the long-term soil pH requires changing the balance of acids and bases in both the soil water and on the cation exchange sites of soil clay surfaces. ServiTech uses the results of a “buffer pH” to calculate ag lime requirements. Other lime requirement methods are available, like titration or a second “doublebuffer” measurement, but they tend to be more expensive or me-consuming.
The “single buffer” pH measurement is used to estimate the amount of reserve acidity in a soil sample. There are several single buffer methods, each used in different regions with specific soil conditions.
The single buffer method requires that the soil pH is measured first in a 1:1 mixture of soil and deionized water. If the pH is below an acceptable level, a buffer solution with an alkaline pH is added to the slurry mixture. The mixture is allowed to equilibrate, and the pH measured a second me.
The alkaline buffer solution will neutralize a portion of the soil acidity during the equilibration step. The difference between the initial buffer solution pH (e.g., pH 7.5) and the second pH measurement aer the soil and buffer solution slurry have equilibrated is then used to calculate the lime requirement.
The greater the difference between the initial pH of the buffer solution and the final pH of the soil-buffer slurry, the greater the amount of reserve acidity, and the greater the ag lime requirement.
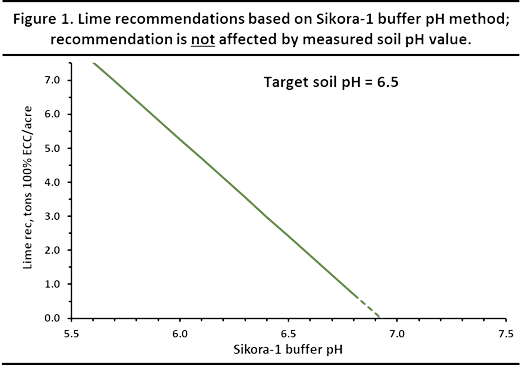
Figure 1 illustrates how the recommended lime rate can be calculated from the single buffer pH value. The lime recommendation calculation is not affected by the measured soil pH value.
¶ Figure 1. Lime Recommendations Based on Sikorsky-1 Buffer pH
Calibration: Ag lime requirements are calibrated to buffer pH values using laboratory incubation studies. The soil pH and buffer pH are measured in a set of soil samples. Various amounts of an alkaline solution are applied to the soil samples. These amounts are equivalent to field ag lime application rates.
The soil pH is measured again aer the samples have been incubated for a month or two. The buffer pH values are calibrated to the differences between the initial and final soil pH values as affected by the alkaline solution rates. The buffer pH value is then used to calculate the equivalent ag lime application rates to reach a target soil pH value.
¶ SMP and Sikora-1 Methods
ServiTech originally used the SMP buffer pH method, the most widely used method in the U.S. at the me. It was originally developed primarily for use in the states of the North Central U.S. The SMP solution contains potentially hazardous materials, so had to be stored and disposed as hazardous waste, an expensive process.
The SMP method has been largely replaced by the Sikora1 buffer pH method, introduced in 2006. The Sikora-1 buffer pH values are essentially the same as SMP buffer pH values. The Sikora solution does not include hazardous materials so does not present a safety hazard.
Limitations: The SMP method was originally designed for soils with significant reserve acidity requiring large amounts of lime (2 tons per acre or more). It was less effective when used to measure pH in:
- poorly buffered soils,
- soils with 10% or greater organic mater, or
- soils with significant levels of kaolinite, aluminum oxides, and iron oxides in their clay fractions.
The SMP method also tended to underestimate lime requirements when the buffer pH was 6.9 or greater. Thus, it was advised not to recommend lime or to extrapolate a lime recommendation with a buffer pH above 6.8, regardless of the measured soil pH. The dashed portion of the line in Figure 1 represents this extrapolated lime requirement.
¶ The Sikora Buffer pH Methods
Impacts on soil pH measurement
A glass electrode is used to make soil pH measurements The Sikora-1 method measures the initial soil pH in a slurry of one- part soil and one-part deionized water (e.g., “1:1 Soil-Water”). This is option referred to as the “water pH” method.
The soil water measurement can be affected by the soil sample characteristics. This can produce an “artificially low” pH measurement, but not affect the buffer pH measurement. The result can be an acid soil with a zero lime requirement.
Soil texture effects: It is more difficult for the pH electrode to stabilize in poorly buffered, low CEC soils resulting in more variable pH results. This low buffering capacity is typical in sandy soils, often found in the Great Plains. It also occurs in soils dominated by certain clay minerals, like those in the Southeast U.S. states.
Soluble salt effects: The soil pH using deionized water can be variable, depending on field conditions that may affect the soluble salt levels. The pH of a soil with elevated levels of soluble salts can be lower than the pH of the same soil with lower soluble salt levels.
Soluble salts can displace H+ and Al3+ from the soil clay exchange surfaces into the deionized water-soil solution during the pH measurement process. This affects the pH electrode causing an apparent increase of the solution acidity, resulting in a lower pH result.
Drought effects: Soluble salts tend to increase as the soil dries and tend to decrease with rain or irrigation. The soil pH measurement can vary from year-to-year and even within the year (e.g., from fall to spring) depending on the relative soluble salt level.
Figure 2 shows the impact of a 2008 drought in Kentucky on soil pH levels. It compares the average soil pH in counties affected by the drought compared the Palmer Drought Index for that county. The declining soil pH values are largely due to soluble salt accumulations in the droughted soils. The more severe the drought, the lower the soil pH as measured in 1:1 soil:water.
¶ Figure 2. Average County Soil pH in Kentucky
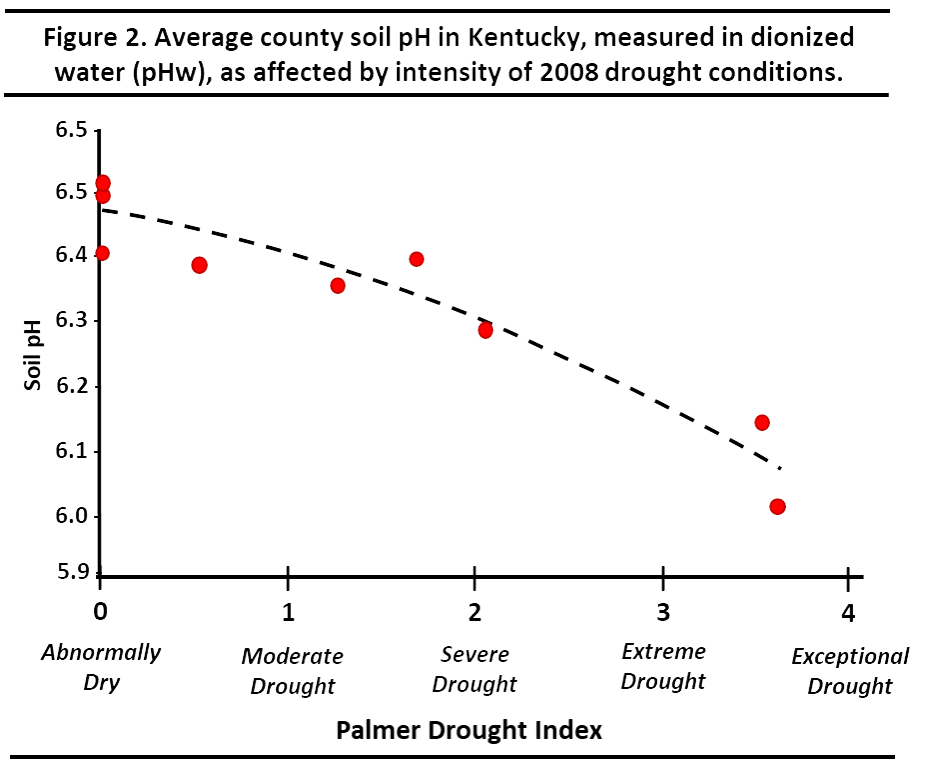
When drought conditions prevail in an area, we have commonly noted soluble salt results to be 0.3 to 0.5 mmho/cm higher than normal. These elevated salt levels are often accompanied by soil:water pH readings that are 0.4 to 0.5 units lower than normal.
Fertilizer application: .Fertilizer materials must dissolve in the soil water to be available for root uptake. This causes soluble salt levels to increase for a me aer fertilizer is applied. The increased soluble salt levels may then affect the soil pH measurement.
Figure 3 compares the effect on measured pH from calcium chloride solutions that provide an increasing soluble salt level. The arrow represents the calcium chloride salt concentration equivalent to an application of 150 lb/ac of nitrogen (N) with170 lb/ac of potash (K2O). The salt effect of this application artificially depressed the soil pH by about 0.7 units.
¶ Figure 3. Potential Effect of Fertilizer Application to Soil pH
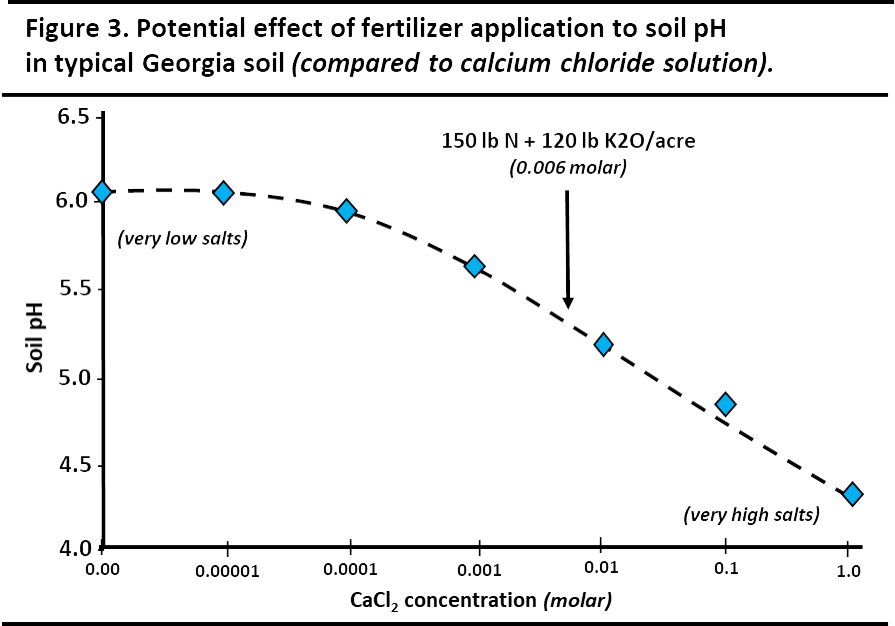
In these situations, the soluble salt effect on soil pH may trigger a lime recommendation, even though it is not needed.
¶ Sikora-2 method: soil pH measurement
The soluble salt effect can be masked by measuring the soil pH in a mild salt solution rather than a soil:water mixture. This requires using standard amount of salt that is slightly greater than the expected saltiest level for most situations. For example, some laboratories use a calcium chloride solution (0.01 M CaCl2) which yields a pH measurement about 0.6 units lower than the soil:water method.
¶ The Sikora Buffer pH Methods
The Sikora-2 method uses a potassium chloride solution (1 M KCl) which offsets the soil pH depression from the soluble salt accumulations that may occur during drought conditions or due to fertilizer applications. It also helps the pH electrode to stabilize more effectively when testing sandy soils.
The initial pH measured in this KCl solution is about 0.9 units less than soil:water pH. We back-calculate this pH reading and report an equivalent soil-water pH result (i.e., “1:1 (c) Water-Soil”). This provides consistency for producers and crop advisers to maintain soil test histories. Sikora-2 method: ag lime recommendation
Options: The Sikora-1 method seemed to have the same challenges as the SMP method for estimating lime requirements in poorly buffered soils. We tried using other single-point buffer pH methods, like the AdamsEvans and Woodruff buffer. They were developed for use in the South and Southeastern U.S. states that often have poorly buffered (low CEC), low organic mater soils.
We found that these two methods seemed to be more effective on sandy soils, but they were also formulated with hazardous materials requiring special storage and disposal.
Another option we considered was to use a two-step “double-buffer” method for sandy soils. This approach uses the measured difference of results between two different buffer solutions to better identify the amount of acidity for an individual soil sample. However, existing double-buffer methods were me consuming and not practical for routine, rapid-turnaround soil testing.
Sikora-2, two-point method: The Sikora-2 method, introduced in 2014, uses two measured points – the soil pH and the buffer pH – to better estimate the amount of reserve acidity and to calculate a lime recommendation for the specific soil sample. This method provides the same benefit that would be obtained with double buffer methods, but is not me consuming.
This two-point lime recommendation method allows the user to identify any target soil pH and then calculates an initial lime requirement from the buffer result. The requirement is then further adjusted by the measured soil pH of the individual sample.
Figure 4 illustrates how the various lime recommendations would be calculated for different combinations of buffer pH and soil pH, all with a target soil pH of 6.5. This compares to the single point method, based only on the buffer pH value, that is illustrated in Figure 1.
¶ Figure 4. Lime Recommendations Based on Sikorsky-2
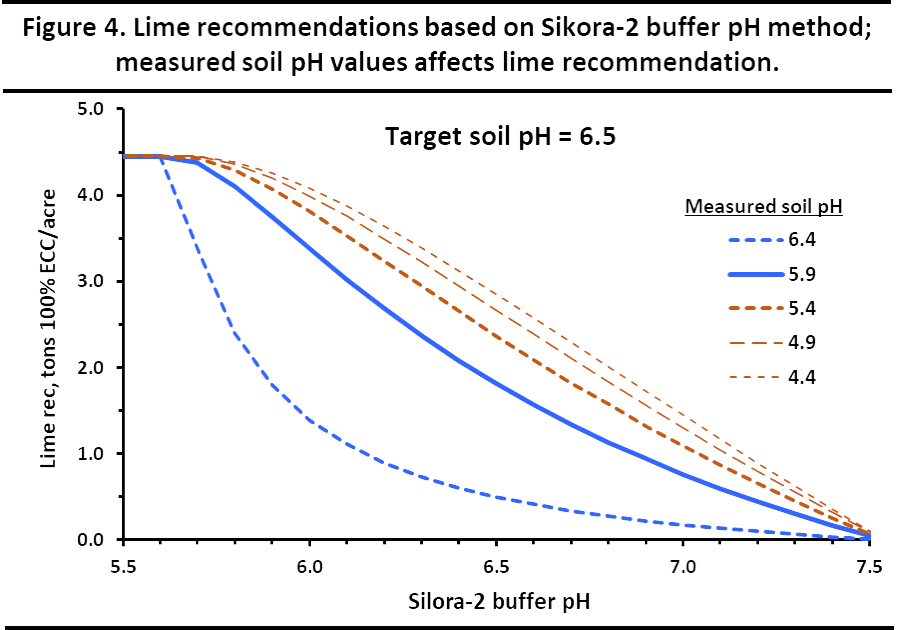
The Sikora-2 method has seemed to work effectively with both our sandy, low CEC soils and with our medium textured to fine-textured soils. The Sikora-2 soil pH and buffer pH reach stability quicker than the pH measured with deionized water in soils with low buffering capacity (e.g., sandy soils) or soils with elevated soluble salts. This allows for better analytical quality, more efficient laboratory operation, and improved ag lime recommendations.
¶ References
Harrold. 2011. Buffer pH methods. Personal communication.2 pg.
Harrold. 2011. Buffer pH methods. Personal communication.2 pg.
Kissel & Vendrell. 2012. Circular 875: Soil pH and Salt Concentration.
Univ. of Georgia Coop. Ext., Athens GA. 2 pg.
Sikora, F.J. 2006. A buffer that mimics the SMP buffer for determining lime requirement of soil. Soil Sci. Soc. Am. J. 70:474-486.
Sikora & Howe. 2010. “A New Method for Testing Soil pH”. IPM Training School, 03 March 201,0 Univ. of Kentucky, Coop. Ext. Serv. https://slideplayer.com/slide/5793692/ accessed 20 Feb 2019 Sikora, F.J. 2012. Double-Buffer Methods Revisited with Focus on Ionic Strength and Soil/Solution Ratio. Soil Sci. Soc. Am. J. 70:474-486.
Sikora, F.J. 2014 “Sikora-2 Buffer for Lime Requirement” in Soil Test Methods From the Southeastern United States. Southern Coop. Series Bulletin No. 419. Southern Ext. & Research Activity Info. Exchange Group – 6. Clemson Univ., Clemson SC.
Peters, Nathan, & Labowski. 2015. "Chapter 4: pH and Lime Requirement (Revised August 2012)" in Recommended Chemical Soil Test Procedures for the North Central Region, Rev. August 2015. Pub. SB 1001. Missouri Agric. Expt. Sta., Univ. of Missouri, Columbia MO.